8.2: Physical Properties and Important Common Names
- Page ID
- 45194
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
- memorize the common names for vinylic and allylic groups including isoprene and styrene
- predict the relative physical properties of alkenes
Common names
The carbon atoms sharing the double bond can be referred to as the "vinyl carbons". The carbon atoms adjacent to the vinyl carbon atoms are called "allylic carbons". These carbon atoms have unique reactivity because of the potential for interaction with the pi bond.
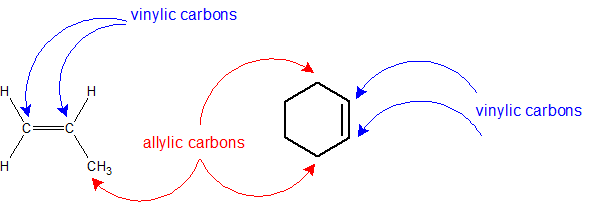
Overall, common names remove the -ane suffix and add -ylene. There are a couple of unique ones like ethenyl's common name is vinyl and 2-propenyl's common name is allyl that need to be memorized.
- vinyl substituent H2C=CH-
- allyl substituent H2C=CH-CH2-
- allene molecule H2C=C=CH2
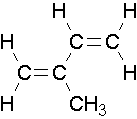
- isoprene is shown below
Physical Properties of Selected Alkenes
Some representative alkenes—their names, structures, and physical properties—are given in the table below.
| IUPAC Name | Molecular Formula | Condensed Structural Formula | Melting Point (°C) | Boiling Point (°C) |
|---|---|---|---|---|
| ethene | C2H4 | CH2=CH2 | –169 | –104 |
| propene | C3H6 | CH2=CHCH3 | –185 | –47 |
| 1-butene | C4H8 | CH2=CHCH2CH3 | –185 | –6 |
| 1-pentene | C5H10 | CH2=CH(CH2)2CH3 | –138 | 30 |
| 1-hexene | C6H12 | CH2=CH(CH2)3CH3 | –140 | 63 |
| 1-heptene | C7H14 | CH2=CH(CH2)4CH3 | –119 | 94 |
| 1-octene | C8H16 | CH2=CH(CH2)5CH3 | –102 | 121 |
Polarity and Physical Properties
Alkenes are non-polar hydrocarbons. The dominant intermolecular forces shared by alkenes are the London dispersion forces. These interactions are weak and temporary, so they are easily disrupted.
Physical States: The physical states reflect the weak attractive forces between molecules. Ethene, propene, and butene exist as colorless gases. Alkenes with 5 to 14 carbons are liquids, and alkenes with 15 carbons or more are solids.
Density: Alkenes are less dense than water with most densities in the range of 0.6 to 0.7 g/mL. Alkenes float on top of water.
Solubility: Alkenes are virtually insoluble in water, but dissolve in organic solvents. The reasons for this are exactly the same as for the alkanes.
Boiling Points: The boiling point of each alkene is very similar to that of the alkane with the same number of carbon atoms. Boiling points of alkenes depend on more molecular mass (chain length). The more intermolecular mass is added, the higher the boiling point. Intermolecular forces of alkenes gets stronger with increase in the size of the molecules. In each case, the alkene has a boiling point which is a small number of degrees lower than the corresponding alkane. The only attractions involved are Van der Waals dispersion forces, and these depend on the shape of the molecule and the number of electrons it contains.
| Compound | Boiling points (oC) |
| Ethene | -104 |
| Propene | -47 |
| Trans-2-Butene | 0.9 |
| Cis-2-butene | 3.7 |
| Trans 1,2-dichlorobutene | 155 |
| Cis 1,2-dichlorobutene | 152 |
| 1-Pentene | 30 |
| Trans-2-Pentene | 36 |
| Cis-2-Pentene | 37 |
| 1-Heptene | 115 |
| 3-Octene | 122 |
| 3-Nonene | 147 |
| 5-Decene | 170 |
Melting Points: Melting points of alkenes depends on the packaging of the molecules so the stereochemistry of the carbon-carbon double bond has a strong influence on the relative melting points. Alkenes have similar melting points to that of alkanes, however, in cis isomers molecules are package in a U-bending shape, therefore, will display a lower melting points to that of the trans isomers. This effect is notable when comparing the melting points of fats and oils. The differences in the melting points is strongly influenced by the long hydrocarbon tails. Oils have a greater number of cis double bonds and exist as liquids at room temperature. Whereas, fats are primarily saturated and exist as solids at room temperature.
| Compound | Melting Points (0C) |
| Ethene | -169 |
| Propene | -185 |
| Butene | -138 |
| 1-Pentene | -165 |
| Trans-2-Pentene | -135 |
| Cis-2-Pentene | -180 |
| 1-Heptene | -119 |
| 3-Octene | -101.9 |
| 3-Nonene | -81.4 |
| 5-Decene | -66.3 |
Exercise
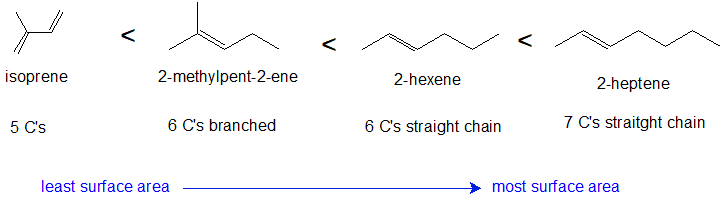
1. Draw the bond-line structures for the following compounds in order of increasing boiling point: 2-methyl-2-pentene; 2-hexene; isoprene; 2-heptene.
2. Which phase will contain the most 3-octene?
a) water or hexane
b) water or benzene
c) methanol or 1-octanol
- Answer
-
1. relative boiling points
2. a) hexane (Hydrocarbons are hydrophobic and lipophilic.)
b) benzene (Hydrocarbons are hydrophobic and lipophilic.)
c) 1-octanol (Hydrocarbons seek the solvent with the most carbons and fewest polar groups.)
Contributors
- Trung Nguyen
- Jim Clark (Chemguide.co.uk)
- Layne A. Morsch (University of Illinois Springfield)

