5.2: Griseofulvin
- Page ID
- 285457
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
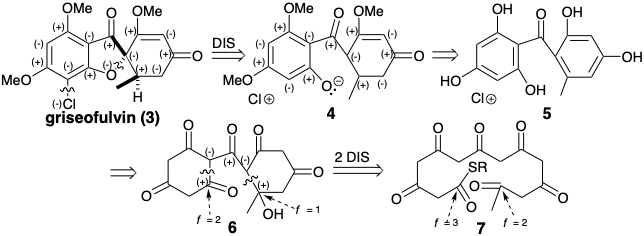
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Polar reactivity analysis of griseofulvin (3) reveals dissonant circuits involving the chloro substituent and furan oxygen. A dislocation cleaving these dissonant circuits suggests an entirely consonant precursor 4 or the aromatic close relative 5. This disconnection -- between a common atom, the spiro carbon, and a non common atom, the furan oxygen -- also leads to major topological simplification. Disconnection of the rings ring in 5 to generate an acyclic precursor must be preceded by conversion of ring C=C bonds into C-C bonds. Thus, addition of water or tautomerization gives a precursor 6 in which polar disconnection of C-C single bonds is feasible. The functionality level at the electrophilic centers in the acyclic precursor 7 suggested by this polar disconnection must be one unit higher than in the intermediate 6 if the cyclization of 6 is to yield 7 directly.
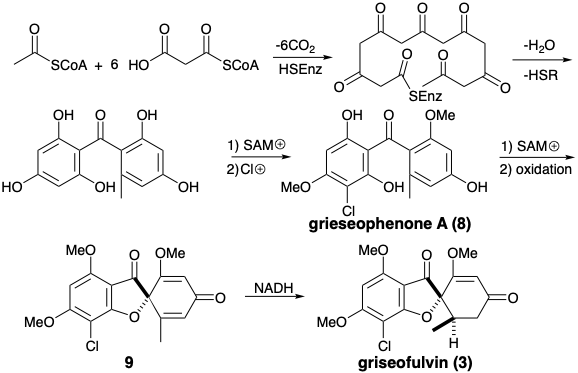
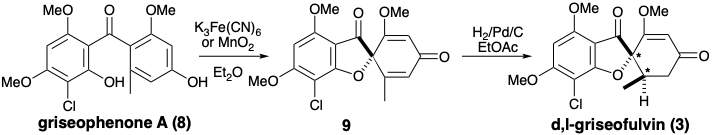
The biosynthesis of griseofulvin (3) illustrates the involvement of electrophilic aromatic substitution and oxidative coupling in the transformation of a poly-β-ketomethylene chain into a functionally and skeletally complex acetogenin. Thus, intramolecular aldol condensations of an enzyme- bound 3,5,7,9,11,13-hexaketohexadecanoic acid thioester followed by hydrolysis, O-methylation with S-adenosylmethionine (SAM) and electrophilic aromatic chlorination generates an intermediate, griseophenone A (8). A dissonant connection in the furan ring of griseofulvin is created by an oxidative coupling that generates dehydrogriseofulvin (9) from 8. Stereo-selective reduction of 9 with NADH then delivers griseofulvin (3).
A Biomimetic Synthesis of Griseofulvin
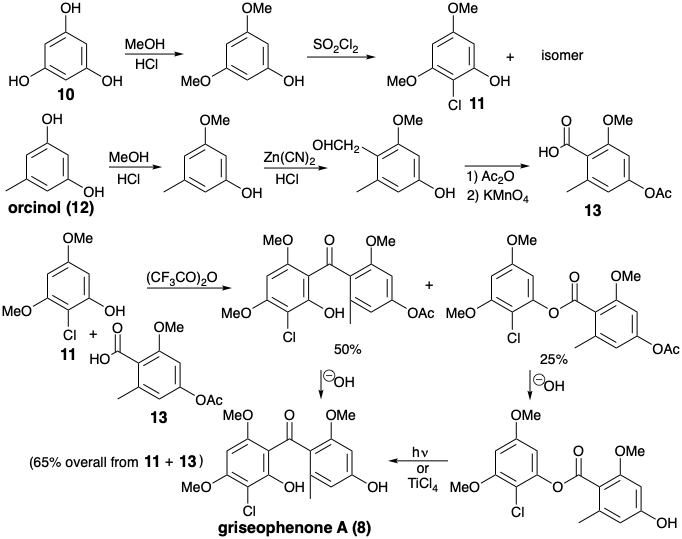
A ferricyanide-induced oxidative coupling of griseophenone A (8) to dehydrogriseofulvin (9) with was exploited in a biomimetic total synthesis of racemic griseofulvin.2 The symmetrical starting materials, phloroglucinol (10) and orcinol (12) were elaborated into the intermediates 11 and 13 by well precedented electrophilic aromatic substitutions. Acylation of 11 with 13 occurred mainly at carbon. The O-acylation product was readily rearranged to the C-acylation product, thus affording griseophenone A (8) in good overall yield. Conversion of 8 to d,l-griseofulvin (3) closely paralleled the biosyn-thetic pathway.
Polar Cyclohexenone Annelation Strategies for Griseofulvin
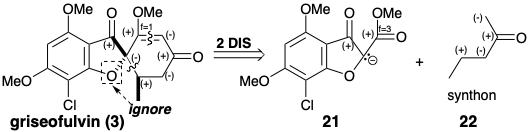
A strategy for the total synthesis of griseofulvin is suggested by a polar analysis that ignores the polar activation afforded by the furan oxygen. Disconnection of two bonds to a common atom, the spiro carbon, in 3 leads to major topological simplification, and suggests a nucleophilic precursor synthon 14 and a biselectrophilic precursor synthon 15. The eneyne 16 is a synthetic equivalent of 15 that should provide 3 directly because 1,4-addition of a nucleophile will decrease the unsaturation level of each electrophilic center by one unit.
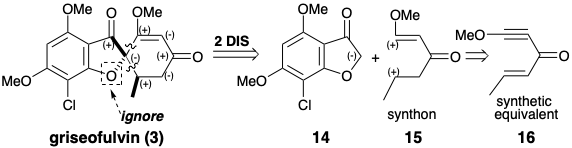
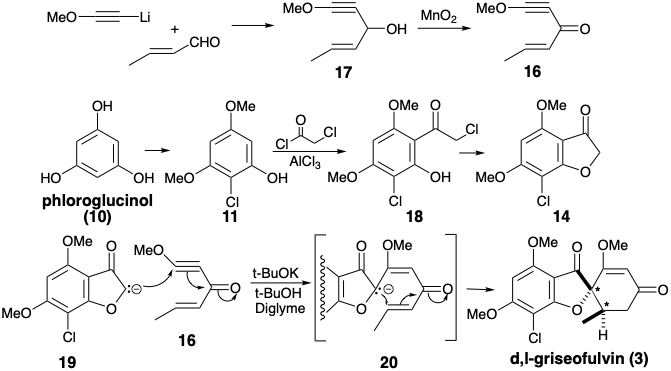
The biselectrophile 16 was prepared from methoxyacetylene and crotonaldehyde, and the precursor 14 of the requisite dissonant nucleophile was obtained from phloroglucinol (10) via 18 produced by acylation of 11 with chloroacetyl chloride. It should be noted that the dissonance in the furan ring of 14 is derived from the dissonant precursor chloroacetyl chloride. Treatment of a mixture of 14 and 16 with base delivered 3 via anions 19 and 20.3
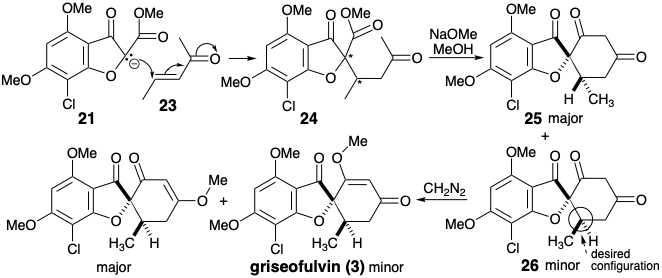
A different strategy is also suggested by a polar analysis that ignores the polar activation afforded by the furan oxygen. Disconnection of two bonds of the cyclohexenone ring as in 3 suggests a well precedented annelation of cyclohexanediones that is similar to a Robinson annelation (see section 4.7). In contrast to the previous strategy, a methyl enol ether is not produced directly. The enone 23 serves as synthetic equivalent for the synthon 22.
Condensation of 21 with 23 gives two diastereomeric cyclohexanediones 25 and 26. This synthesis is less efficient than the previous one because the major diastereomer 25 is epimeric with the natural product 3 and methyletherification of the minor diastereomer 26 occurs with unfavorable regioselectivity.4
A Cycloaddition Strategy for Griseofulvin
Danishefsky's strategy for a total synthesis of griseofulvin5 was designed around his method for cyclohexenone annelation through Diels-Alder reaction of highly oxygenated dienes, e.g., 27.
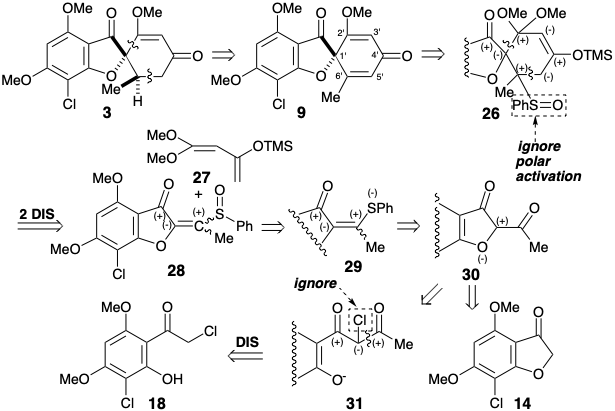
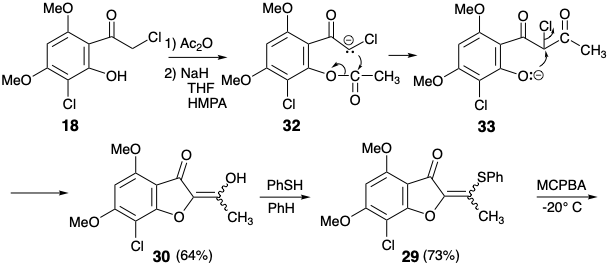
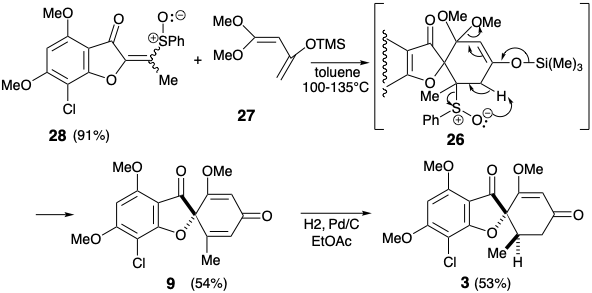
This boundary condition guides and channels the retrosynthetic analysis to seek a two-bond disconnection of the cyclohexenone ring to a butadiene and dienophile precursor. Furthermore, the stereoselective conversion of 9 to give 3 established previously2 was adopted to simplify the stereochemistry of the target. The dislocation to 9 removes one asymmetric center, that can be introduced at the end of the synthesis by stereoselective hydrogenation. For the desired Diels-Alder reaction, a C=C bond between carbons 3' and 4' is required. Therefore, the 2',3' and 5',6' C=C bonds in 9 must be generated after the key Diels-Alder step. The requisite 3',4' C=C bond is provided by dislocation of 9 to the enol-keta1 derivative 26. The 5',6' C=C bond could be introduced in 26 by a variety of elimination processes. The choice of a sulfoxide as leaving group is dictated by the additional utility of the sulfoxide group for activating the dienophile 28 toward Diels-Alder reaction with the diene 27 which is necessarily electron-rich. The sulfoxide can also be expected not to control the structural selectivity of the Diels-Alder reaction, that will be controlled instead by the carbonyl group of the furanone ring in 28. The electron withdrawing sulfoxide group is dissonant with respect to the furanone carbonyl in 28, but it can be obtained by oxidation of the corresponding electron donating sulfide group in 29. This consonant vinyl sulfide is simply an enol sulfide derivative of the dione precursor 30. This dione might be available by acylation of the furanone 14 that was used in a previous synthesis of griseofulvin. Alternatively, construction of the dissonant C-O bond in 30 could be achieved after completion of the carbon skeleton but a nucleofuge would be required in 31 because the carbonyl groups can not provide the requisite electrophilicty. Ignoring the chloro group in 31, the 1,3-dicarbonyl array is consonant and can be constructed by Claisen acylation of the ketone 18 that was also used in the previous stereoselective synthesis discussed above.
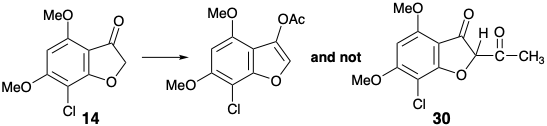
In fact, O-acylation of the enolate anion from 14 occurs to the complete exclusion of carbon acylation required to produce 30. On the other hand, an intramolecular delivery of the acetyl electrophile in 32 served to unmask Intramolecular O-alkylation of the intermediate phenolate 33 delivered the desired dione 30 that exists as an enol tautomer. Conversion of the enol sulfide 29 from 30 into the corresponding sulfoxide was accomploshed by selective oxidation of the sulfide in the presence of a C=C bond with MCPBA at low temperature. Diels-Alder addition of the resulting vinyl sulfoxide 28 to the 1,3-diene 27 was followed, in situ, by thermal elimination of phenylsulfenic acid and methoxytrimethylsilane from an intermediate cyclohexene 26 to deliver cyclohexadienone 9.