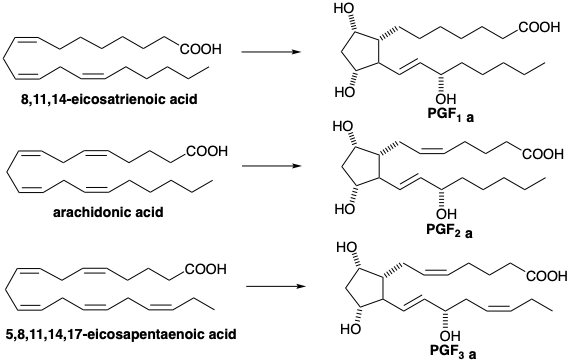
3.2: Biosynthesis of Prostaglandins
- Page ID
- 285443
Prostaglandins are the skeletally most complex molecules we have yet considered. All prostaglandins are 1,3-dioxygenated cyclopentane derivatives with a 7-carbon carboxylic acid side chain and a vicinal 8-carbon γ-hydroxy- vinyl side chain. Three series of prostaglandins are known which are exemplified by prostaglandins F1α, F2α, and F3α. These are designated PGF1α, PGF2α, and PGF3α and respectively have one,two, or three C=C bonds in the side chains. The prostaglandins are appropriately considered at this point since their biosynthesis from tri-, tetra-, or pentaenoic fatty acids is very simple, involving the formation of only one new C-C bond. Moreover, the plethora of strategically different syntheses of prostaglandins which have been achieved in the laboratory offer a unique opportunity to gain an appreciation for the myriad solutions to the problem of planning a complex molecular synthesis.
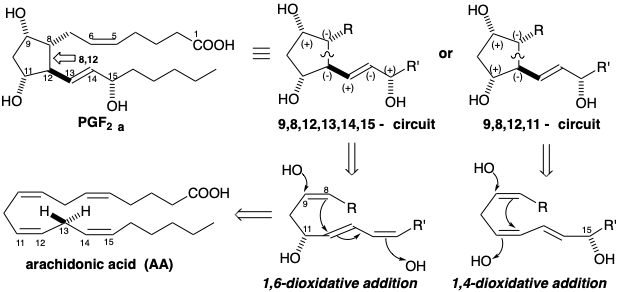
With the exception of a bridge between carbons 8 and 12, the topology -- an unbranched chain of twenty carbons -- as well as the terminal carboxyl functionality of prostaglandins suggests fatty acids as biosynthetic precursors. Polar reactivity analysis of prostaglandin F2α (PGF2α) reveals that the 8,12-bond lies on a dissonant circuit between the hydroxyl groups on carbons 9 and 11 (the 9,8,12,11-circuit) and a dissonant circuit between the hydroxyl groups on carbons 9 and 15 (the 9,8,12,13,14,15-circuit). The 8,12-bond cannot be formed in a polar reaction involving polar activation by any two target related functional groups. The observed dissonant functionality pattern could be generated by 1,4-dioxidative addition of two hydroxyls to a 15-hydroxy fatty acid precursor or by 1,6-dioxidative addition of two hydroxyls to an 11-hydroxy fatty acid precursor. The requisite hydroxy fatty acid precursors might reasonably be produced by allylic oxidation, e.g. of arachiconic acid (AA), involving hydrogen removal from position 13 and hydroxyl addition at position 11 or 15.
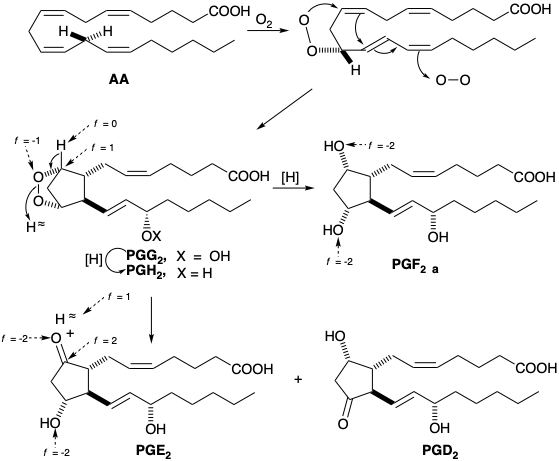
In nature, prostaglandins arise by an oxidative cyclization of poly-unsaturated twenty-carbon fatty acids, which begins with enantiospecific removal of the L-hydrogen atom of the prochiral methylene group at C-13 coupled with enantiospecific introduction of oxygen at the allylic C-15 position. Subsequent cyclization and termination by addition of a second molecule of oxygen leads to a 15-hydroperoxy bicyclic peroxide (PGG), that is reduced to a 15-hydroxy bicyclic peroxide (PGH). These intermediates, known as prostaglandin endoperoxides, have been isolated and shown to yield prostaglandins. Reduction of the peroxy bridge gives PGF, while disproportionation gives β-hydroxy ketones PGE and PGD. The carbons in prostaglandins are numbered one to twenty starting at the carboxyl carbon and following the numbering system of the biosynthetic precursor fatty acids.
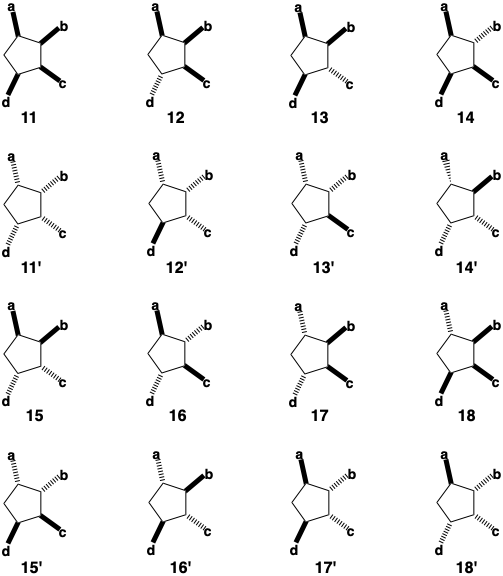
Important features of the biosynthesis of prostaglandins are its dia-stereo- and enantioselectivity. Consider the possible stereochemical relationships between the four substituents on the cyclopentane ring of PGF2α. There are 2n different possible arrangements for n stereocenters each having two possible configurations. Of the sixteen possible stereoisomeric arrangements, only 13' is found in the natural product. The isomers 11-18 are diastereomers. They possess unique stereochemical interrelationships of their four substituents. Thus, they are stereoisomers that have different configurations at one or more (but not all) of their stereocenters and, therefore, are not mirror images of each other. The remaining isomers 11'-18' are mirror images or enantiomers of the other isomers. Taking into account a fifth stereocenter at position 15 in the sidechain, there are 32 possible stereoisomers of PGF2α, two enantiomeric sets of 16 diastereomers. The biosynthesis is completely stereoselective. For any synthesis of a complex molecule, this is important because the less stereoselective a synthesis, the lower the yield of the desired product. Also, purification of the product is usually difficult if it is contaminated by stereoisomers since these often possess chemical and physical properties that are very similar to those of the desired isomer. In the biosynthesis of PGF2α, the first stereocenter, that at position 11, is introduced enantioselectively by the action of an asymmetric reagent (enzyme) on a prochiral precursor generating only one enantiomeric intermediate. Such a process, known as asymmetric induction, is inherently more efficient than a synthesis involving separation of a racemic mixture of enantiomeric products (resolution) since no starting materials are wasted in the generation of wrong isomers. The biosynthetic strategy for PGF2α involves a connection that ties the two ring hydroxyl groups together in a temporary bridge. The latter serves as a stereocontrol element assuring a cis relationship between the hydroxyls at positions 9 and 11, and allows the introduction of both cyclopentane oxygens atoms as a single molecule of oxygen.
There are three topologically unique strategic categories for the synthesis of prostaglandins: (a) syntheses from acyclic precursors, (b) syntheses from multicyclic precursors by cleavage of temporary bridges, and (c) syntheses from precursors containing an isolated cyclpentane ring. Examples of each strategic type will be considered in the following three sections.


