3.1: Biosynthesis of Fatty Acids
- Page ID
- 285442
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
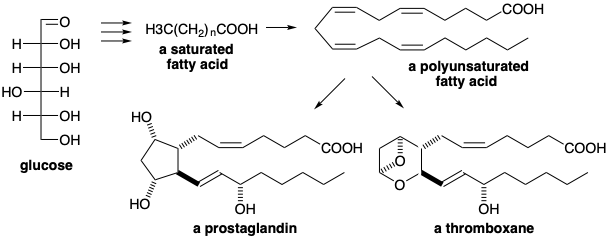
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Fatty acids have larger carbon skeletons than sugars, but they are structurally simple. They have long unbranched chains of carbon atoms with much less functionality than sugars and no centers of asymmetry. Thus, they are more reduced (less oxygenated) than sugars. Higher animals have only limited capacity for storage of sugars as polysaccharides. Therefore, sugars are converted into fatty acids that may be stored as triesters of glycerol and used in the biosynthesis of the complex polar lipids of membranes and in the biosynthesis of prostaglandins and thromboxanes, two large groups of physiologically active substances.
A plausible strategy for the biosynthesis of stearic acid, which consists of an eighteen-carbon chain, involves reductive deoxygenation of a highly oxygenated precursor 1 that could be assembled by polar union of three six-carbon sugar molecules (see below). Such a strategy would economically utilize all of the carbon atoms in glucose to build the skeleton of the target. The carbonyl group in fructose 6-phosphate (F6P) activates position 1 toward alkylation by carbon 6 in F6P or glucose 6-phosphate (G6P). The nucleophilic substitution at position 6 would displace the activating oxygen from two precursor sugars delivering the precursor 1 that lacks oxygen at positions 6 and 12. Removal of twelve remaining hydroxyl and two carbonyl groups and oxidation of the terminal aldehyde in the precursor 1 to provide the carboxyl of stearic acid would require eighteen equivalents of hydride from e.g. NADPH. The only byproduct from such a synthesis would be eighteen molecules of water. But Nature not only uses glucose as the source of carbon but, except for the photosynthetic generation of NADPH, also uses glucose as the ultimate source of hydride for all organic biosynthesis.
The actual biosynthetic strategy is an ingenious process that co generates all the reducing agent, NADPH, required for deoxygenation, by oxidation (hydride abstraction) from an aldehyde intermediate derived from glucose. The byproducts from the biosynthesis are eighteen equivalents of water as well as nine equivalents of a byproduct \(\ce{CO2}\). Instead of three molecules of glucose, which would be required for the first strategy, the actual biosynthesis consumes four and a half molecules of glucose for every molecule of stearic acid produced. The carbons that are incorporated into the fatty acid product are almost all reduced while those in the \(\ce{CO2}\) byproduct have been oxidized.
A boundary condition governing the biosynthetic strategy for fatty acids is that the reagents and reactions should be readily adapted to the biosynthesis of a large selection of fatty acids with differing chain lengths. This suggests a repeatable chain-growing strategy: addition of a two-carbon carboxylic (acetic) acid to the growing fatty acid chain by a Claisen condensation. Thus, if the strategy is to be repeatable, a shorter chain carboxyl will serve as electrophile and become a keto group after C-C connection by polar bond formation with a two-carbon carboxyl-stabilized nucleophile on the α-carbon, then the functionality level of the electrophile (f = 3 for a carboxyl) will become (f = 2 for a ketone) in the resulting β-keto group derived from the carboxyl group in a precursor 2b that incorporates two less carbons.
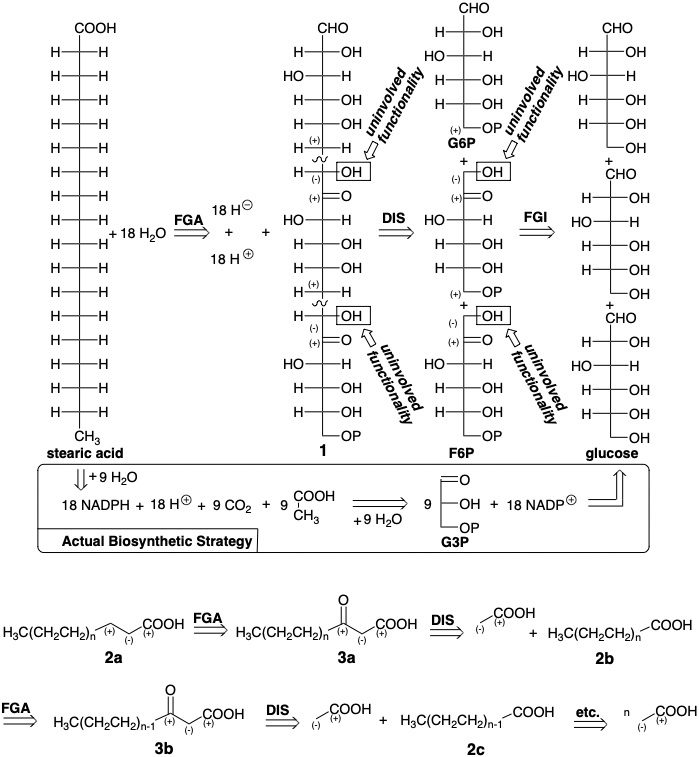
Further refinement of this strategy recognizes the need for a better leaving group than hydroxyl, especially considering that most of the carboxyl group will be in the form of carboxylate at physiological pH. This suggests replacement of the hydroxyl, functional group interconversion (FGI), in 2a and 3a with a better leaving group indicated by X in 4a and 5a.

In the biosynthesis, all of the carbon atoms of a fatty acid are derived from acetyl CoA, that transfers its acetyl group to the thiol group of an a protein-bound acyl carrier protein (ACP). Both acetyl-S-ACP and acetyl-S-CoA are thioesters, acylating agents corresponding to \(\ce{H3C–COX}\) that are more electrophilic than their oxygen analogues because back donation of electron density of the nonbonding electrons from the large sulfur orbitals is far less important than back donation of nonbonding electrons from ester oxygen whose orbitals overlapp more effectively with those of the carbonyl π-bond.
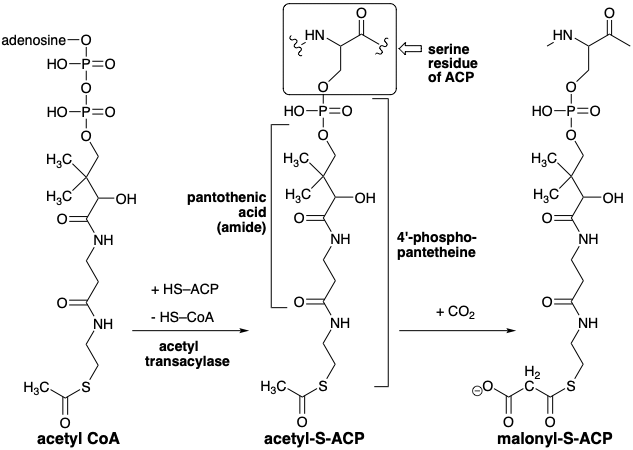
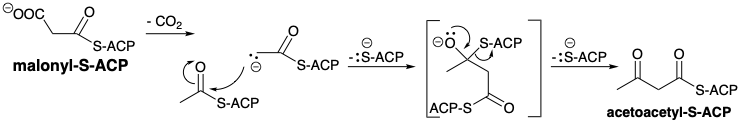
A key feature in the biosynthesis of fatty acids is a requirement for carbon dioxide as bicarbonate ion, although the \(\ce{CO2}\) or \(\ce{HCO3-}\) is not incorporated into the fatty acids. This suggests a strategy in which \(\ce{CO2}\) is temporarily added to a precursor and then eventually removed after it has served its purpose. In fact, except for two carbon atoms at the alkyl terminus, acetyl-CoA is not the immediate precursor of the fatty acid carbon chain. Rather, an activated form of acetyl CoA, malonyl CoA, is generated by carboxylation of acetyl CoA. The electron deficiency of \(\ce{CO2}\) suggests that its function might be to remove electron density from an anionic intermediate, e.g., its function might be to stabilize a carbanion intermediate such as a carbanion on the α-carbon of an acetyl group. Thus, the nucleophile that reacts with acetyl-S-ACP might be a carbanion derived from malonate 7. Carboxylation of acetyl-S-ACP delivers malonyl-S-ACP.
For this refinement of the retrosynthetic analysis of the β-keto fatty acyl 5a would involve addition of an activating carboxyl group, reactivity control element addition (CEA), to a precursor 6a to facilitate carbanion formation (see page 44). Thus, the last carbon-carbon bond forming synthetic step is suggested by a dislocation of the precursor 6a involving carbon-carbon bond disconnection (DIS) suggesting a precursor 4b with two less carbons than 5a (three less than 6a). The synthetic strategy suggested by the above retrosynthetic analysis involves Claisen condensation of a malonate carbanion derived from 7 with a precursor 4b with two less carbons than the desired fatty acid 2a followed by decarboxylation of 6a to deliver 5a and accomplish net chain elongation by two carbons.
The carboxylation of acetyl-S-CoA is promoted by acetyl CoA carboxylase and involves transfer of a carboxyl group from an enzyme bound \(\ce{CO2}\) carrier, biotin. The role of carboxybiotin in thie biosynthesis deserves further consideration. It delivers anhydrous \(\ce{CO2}\) to an active site that, presumably encapsulates the reactants in a water-free environment. In a aqueous environment, \(\ce{CO2}\) is present as bicarbonate that is much less electrophilic than anhydrous \(\ce{CO2}\). Thus, the energy expended, in the form of ATP hydrolysis to ADP, results in the conversion of a weak electrophile into a stronger electrophile. Simultaneously, a base, the biotinyl anion, is produced that is strong enough to abstract a proton from acetyl-S-ACP. This proton abstraction also must occur in an aprotic environment because otherwise water would protonate both the biotinyl anion and the acetyl-S-ACP carbanion.

The fatty acid carbon chain is then assembled by a series of Claisen condensations starting with malonyl plus acetyl. In fact, the CoA derivatives of both synthons are first transformed into enzyme bound thioesters of the acyl carrier protein (ACP). The acyl groups are bound to the protein, located in the cytoplasm, by a 4'-phosphopantetheine ester of a serine residue. The acetyl group is then transferred to a specific cysteine residue of another enzyme of the fatty acid synthetase complex, β-ketoacyl-ACP synthetase (HS-synthetase). The activating carboxyl group in malonyl-S-ACP assures that the methylene of this acetyl synthon acts as the nucleophile. After condensation, the activating group is lost to give acetoacetyl-S-ACP.
There is another reasonable hypothesis for the role of the carboxyl group in malonyl-S-ACP in the condensation with acetyl-S-ACP. Rather than serving as an activating group to facilitate generation of a malonyl carbanion, it may serve as a latent carbanion. Driven by the energy released upon formation of a C=O bond, decarboxylation may generate an acetyl-S-ACP carbanion in an anhydrous environment in an active site of the fatty acid synthetase complex. This strong nucleophile would be acylated by acetyl-S- ACP to form acetoacetyl-S-ACP directly. Thus, the role of the carboxylate group is to allow the generation of a strongly basic carbanion without the requirement for a strong base, and in the absence of water that would protonate the carbanion or a strong base. This putative role of the carboxyl group is related to its role in the carboxylation of acetyl-S-ACP, except that instead of the decarboxylation generating a strong base that abstracts a proton from acetyl-S-ACP to produce the acetyl-S-ACP carbanion, the decarboxylation of malonyl-S-ACP generates the acetyl-S-ACP carbanion directly. In both scenarios, the generation of a strongly basic intermediate is driven by the energy released by the formation of a C=O bond in a water-free environment required to preclude destruction of the strongly basic intermediate.
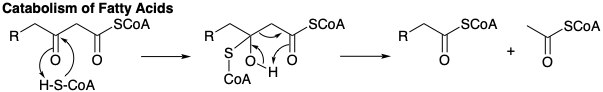
The β-keto group is then reduced to a methylene group in a series of hydride reductions involving NADPH. The first reduction enantiospecifically gives D-β-hydroxy-butyryl-S-ACP under catalysis by β-ketoacyl-ACP-reductase. The β-hydroxy ketone is readily dehydrated under the influence of enoyl-ACP dehydratase to give the α,β-unsaturated thioester, crotonyl-S-ACP. Reduction of the latter occurs via 1,4-addition of hydride from NADPH to the electrophilic β-carbon of this D-2,3-unsaturated ester catalyzed by crotonyl-ACP reductase. The resulting butyryl-S-ACP then condenses with a second malonyl-S-ACP leading ultimately to hexanoyl-S-ACP and so on until seven molecules of malonyl-S-ACP have been combined with one acetyl-S-ACP. The process stops at palmityl-S-ACP from which palmitic acid is released by the action of a hydrolytic deacylase. Further elongation of the carbon chain occurs in the mitochondria by addition of acetyl-S-CoA rather than malonyl-S-CoA. The same enzymes in the mitochondria catalyze the reverse reaction, the catabolism (oxidative degradation) of fatty acids except that hydride reduction of the α,β-unsaturated ester involves NADPH; whereas, the corresponding dehydrogenation steps in the breakdown of fatty acids to acetyl CoA involve a flavoprotein as hydrogen acceptor. Catabolism of fatty acids also differs mechanistically from their anabolism in that acetyl CoA is produced directly in the thiolytic cleavage of a 3-keto fatty acyl-CoA by CoASH. That is, malonyl CoA is not involved in fatty acid catabolism.

Two different pathways exist for the biosynthesis of unsaturated fatty acids. One of these involves aerobic dehydrogenation of palmitic or stearic acid to give palmitoleic or oleic acids respectively. This reaction is remarkable because of its regiospecificity, its stereospecificity, and because the hydrogen atoms removed are remote from any functional group and, therefore, are not activated toward chemical reactions. An interesting feature of the dehydrogenation reaction is the concomitant oxidation of NADPH. The enzyme system is an example of a class of oxygenases which require a coreductant, such as NADPH, known as a mixed function of oxygenases.

A different pathway is operative in anaerobic bacteria. Thus, β-hydroxy-decanoyl- ACP is dehydrated by a specific enzyme, β-hydroxydecanoyl-ACP dehydratase that yields thecis-β,γ(or Δ3)-decanoyl ACP rather than the trans−α,β(or Δ2)-isomer formed in saturated fatty acid biosynthesis. Further elongation by malonyl-ACP then leads to palmitoleic acid. All polyunsaturated fatty acids biosynthesized in animals arise from palmitoleic or oleic acid by further chain elongations or dehydrogenations similar to those described above. Two of these precursor fatty acids, linoleic and linolenic acids, cannot be synthesized in mammals and must be obtained from plant sources; they are therefore called essential fatty acids.

Oxidative degradation (catabolism) of unsaturated fatty acids to acetyl-CoA follows much the same pathway as the corresponding saturated acids. Thus, successive C2 units are removed by thiolytic cleavage of β-ketothioesters.

When a cis-β,γ-unsaturated thioester results (e.g. 8), it is isomerized under catalysis by the enzyme enoyl-CoA isomerase to the trans-α,β-unsaturated isomer (e.g. 9) that is an intermediate in the biosynthesis. Further degradation then proceeds as usual. Since hydration of a cis-α,β-unsaturated thioester (e.g. 10), promoted by enoyl hydratase, gives a D-3-hydroxyacyl-CoA, epimerization, promoted by 3-hydroxyacyl CoA-epimerase, must occur before further degradation may occur. It is noteworthy that only the L-enantiomer is dehydrogenated to β-ketoacyl-S-CoA in oxidative degradation, while only the D-enantiomer is produced in the reduction of a β-ketoacyl-S-ACP during the biosynthesis of fatty acids.


