1.3: Perception of Structure-Functionality Relationships
- Page ID
- 285430
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Exploitation of functionality in synthetic planning requires an understanding of the interrelationships between chemical reactions and functionality. This is most effectively achieved in terms of basic electronic reaction mechanisms that allow a very compact and systematic classification of hundreds of synthetic reactions. For example, let us systematically consider the relationships which inhere between molecular structure and functionality with respect to polar reactions.
Functionality Level Changes
For organic synthetic analysis it is often assumed that all carbon centers in a hydrocarbon are not activated toward polar C-C bond forming reactions, hence, the name paraffin (from the Latin parum affinis = little affinity) denotes relative unreactivity. The hydrocarbon skeleton of organic molecules is considered to be a homogeneous conglomeration of unreactive carbon atoms. It is useful to define the functionality level9 of an atom as f = the number of valence electrons in the neutral atom minus the number of electrons assigned to the atom by the following protocol: all bonding electrons are divided equally between C-C, C=C, C≡C, C-H, M-M or X-X, but all bonding electrons are given to the better nucleofuge (often but not always the more electronegative atom, vide infra) for C-X, C-M, or X-Y bonds, where X and Y are hetero atoms, and M is a metal. Thus, for example, the electrons taken or given to carbon when breaking the following bonds to carbon are assigned as follows: RO-, RS- (-1); O= (-2); F-, Cl-, Br-, or I- (-1); R2N-, R2P- (-1); RN= (-2); N≡ (-3); Li-, Na-, K-, R2Al-, R3Si- (+1).
The functionality level approximation emphasizes the similarity between similarly functionalized carbons. Thus, the functionality level of all carbons in a hydrocarbon is zero. That is, in a hydrocarbon all carbon atoms whether quaternary, tertiary, secondary, or primary, share a common functionality level (f = 0). Likewise, all carbinol carbon atoms share a common functionality level (f = +1) regardless of whether they are primary, secondary or tertiary. Aldehydes and ketones share a common functionality level (f = +2). To organic chemists these conclusions of similarity are tacitly accepted. The functionality level approximations differ from those made for defining the oxidation state x = the number of valence electrons in the neutral atom minus the number of electrons assigned to the atom by the following protocol: all bonding electrons are divided equally between C-C, C=C, C≡C, M-M or X-X, but all bonding electrons are given to the more electronegative element for bonds between different elements.10 It is of little significance to an organic chemist that the oxidation states of the carbonyl carbon in formaldehyde (x = 0), other aldehydes (x = +1), and ketones (x = +2) differ as do the oxidation states of primary (x = -1), secondary (x = 0), or tertiary (x = +1) carbinol carbons. The abovementioned contrasts between the oxidation state and functionality level approximations result from the fact that in effect, the functionality level approximation assigns an oxidation number of 0 for hydrogen when bound to carbon in contrast with the oxidation state approximation that assigns an oxidation number of +1.
As we shall see, the functionality level concept is useful in the context of polar reactions which are those that result in bond formation by electron pair donation from an electron rich synthon (nucleophile) to an electron deficient synthon (electrophile). In this context, for example, organic chemists generally consider all methyl halides, i. e. fluoride, chloride, bromide, iodide, to be similarly functionalized, and thus it is appropriate that they all share f = +1. It is of little significance (and probably not widely known) that the oxidation states of the carbons in methyl iodide (x = -4) and methyl bromide (x = -2) are different.
Interconversions of functional groups of different functionality level correspond to oxidations or reductions:
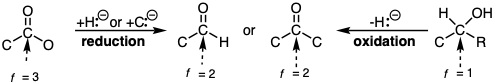
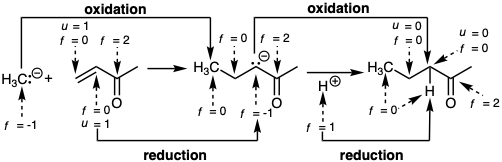
Heterolysis of the C-H bond is viewed as a special mode of C-H reaction that results in functionalization of a hydrocarbon. If hydride is abstracted the reaction is considered oxidation whereas proton abstraction is considered reduction.
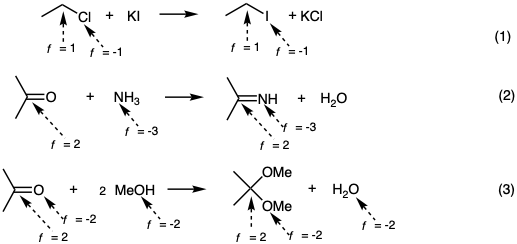
All functional groups of the same functionality level are, in principle, interconvertable by metatheses or polar addition and elimination reactions such as in equations 1-3.
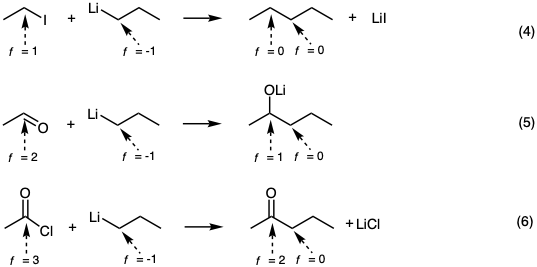
For organic synthetic analysis, it is important to recognize that polar carbon-carbon bond forming reactions are redox processes. The carbon nucleophile is oxidized, and the carbon electrophile is reduced. In terms of functionality levels of carbon in representative hypothetical polar reactions; nucleophilic substitution, nucleophilic addition, and nucleophilic acyl substitution are indicated in equations 4-6 respectively.
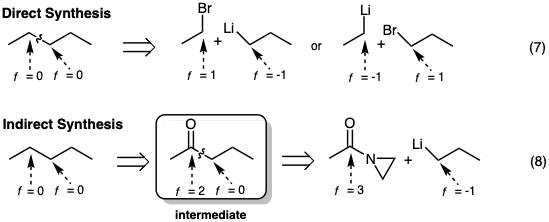
Thus, if we desire a product without functionality (f = 0) we must plan to react a (+1) electrophile with a (-1) nucleophile (equation 7). Alternatively, we may use reactants of other functionality level, but the functionality level of the initial product will have to be changed (oxidation or reduction) after C-C bond formation (e.g. equation 8).
Unsaturation Level Changes
If carbon functionality is defined as carbon for which f ≠ 0, then C=C and C≡C bonds are not considered to be functionality. Clearly, then, functionality is not the only molecular characteristic (structural feature) that facilitates bond forming reactions. In order to systematically consider the relationships which inhere between molecular structure and polar reactions, it is useful to define the unsaturation level of a specific atom as u = 0 for all singly bonded atoms, as u = 1 for all atoms involved in homonuclear double bonding, and as u = 2 for all atoms involved in homonuclear triple bonding. 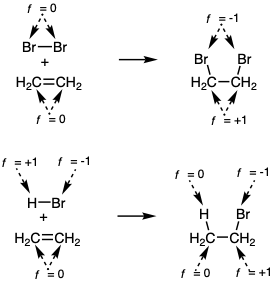 Thus, carbon-carbon and homonuclear multiple bonding in general does not change functionality levels. Introduction of C-C unsaturation is viewed as activation rather than oxidation (increased functionality level). Hydrogenation of C-C unsaturation is viewed as saturation rather than reduction of functionality level. These conventions find analogy in the concepts of coordinative unsaturation in organometallic chemistry. Thus, reactivity depends of f and u. Changes in f during chemical reactions are always balanced, i.e. an increase of f for one atom requires a decrease of f for another atom. For example, addition of bromine to C=C results in oxidation of both C's and reduction of both Br's (from f = 0 to f = -1).
Thus, carbon-carbon and homonuclear multiple bonding in general does not change functionality levels. Introduction of C-C unsaturation is viewed as activation rather than oxidation (increased functionality level). Hydrogenation of C-C unsaturation is viewed as saturation rather than reduction of functionality level. These conventions find analogy in the concepts of coordinative unsaturation in organometallic chemistry. Thus, reactivity depends of f and u. Changes in f during chemical reactions are always balanced, i.e. an increase of f for one atom requires a decrease of f for another atom. For example, addition of bromine to C=C results in oxidation of both C's and reduction of both Br's (from f = 0 to f = -1).
Addition of HBr to C=C results in oxidation of one C (the one receiving Br) while \(H\oplus\) is reduced to H•. The functionality level concept corresponds well with experience for the most part. Although synthetically valuable differences in chemical reactivity are achievable for 1°, 2°, and 3° C-H bonds of hydrocarbons, it is useful to view these as different proclivities towards oxidation (hydride abstraction) or reduction (proton abstraction).
An increase of the functionality level of the carbon nucleophile also accompanies nucleophilic conjugate addition. But the carbon electrophile is not reduced. Rather, \(H\oplus\) is reduced to H• and the unsaturation level of the carbon electrophile is decreased.