1.2: Logic Centered Analysis
- Page ID
- 285429
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Exhaustive Retrosynthetic Analysis
Three things must be established to accomplish the total synthesis of an organic molecule. These are the appropriate (1) carbon network, (2) functionality, and (3) stereochemistry. The carbon network consists of carbon atoms and a set of connections between them. An exhaustive retrosynthetic analysis of the problem of synthesizing a complex organic molecule would include consideration of all possible strategies involving each C-C bond as the hypothetical last connection in the skeletal construction, a disconnection (DIS) in the retrosynthetic analysis. Furthermore, it may be advantageous to generate intermediates that contain bonds not present in the final target. These bonds must be severed at some stage in the synthesis. For example, consider a synthesis of the tetracarboxylic acid 4. This target could be obtained readily by oxidative cleavage of endo-dicyclopentadiene (5), itself readily available by dimerization of cyclopentadiene (6). In planning such a synthesis, these bond cleavages correspond to dislocations which generate connections (CON). Thus, an exhaustive analysis must also consider all possible bond cleavages that could generate the desired skeleton from a more highly connected precursor.

Besides establishing the requisite carbon skeleton from available precursors by the formation or cleavage of C-C bonds, the generation of a synthetic target may require manipulation of functional groups. Thus, synthetic targets may contain functionality that is different than that in a readily available precursor. For example, a well known synthesis of ketones 7 involves oxidation of alcohol precursors 8 that are, in turn, often assembled by the union of aldehydes 9 with Grignard reagents 10. Another example is provided by a strategy for the synthesis of cyclohexane 11. Cyclohexene 12, that is readily available from 13 and 14, is an excellent precursor that would deliver the cyclohexane 11 upon saturation of the C=C bond.

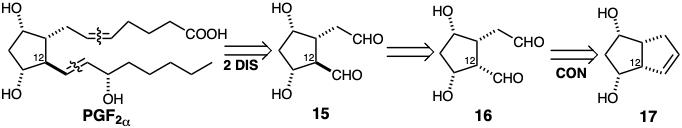
Finally, some reactions neither change the carbon network nor modify functionality, but merely alter stereochemistry. For example, a possible dislocation of the precursor 15 for \(PGF_{2\alpha}\) is epimerization of the stereocenter at position 12 since this allows generation of 16 from a readily available cis fused bicyclic precursor 17.
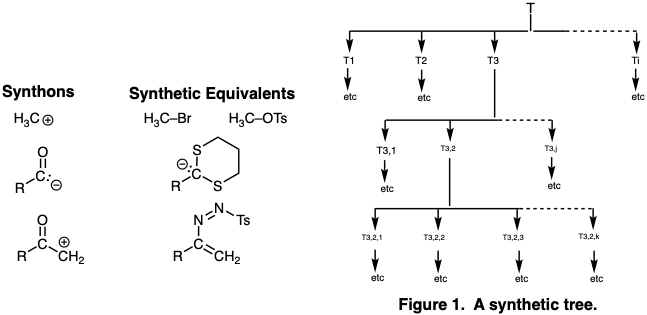
An exhaustive logic centered retrosynthetic analysis would consider every possible disconnection, connection, functional group modification, and stereochemical modification as a potential last step of the synthesis. In so doing, a set of subtarget structures (e.g. T1, T2, T3 . . . Ti) is generated, which may be converted in a single synthetic operation, that is, chemical step, to the synthetic target. The same process is applied to each subtarget and so on until the molecule is reduced to several sets of readily available starting materials, and a complete tree of synthetic intermediates (sometimes referred to as a synthetic tree) is generated (Figure 1). It is often convenient during the planning process to generalize potential intermediates of a particular type. Such generalized intermediates or synthons may correspond to stable organic molecules or to hypothetical reactive fragments such as a "methyl cation", "acyl carbanion" or “α-keto carbocation”. The actual organic intermediates corresponding to various synthons are called synthetic equivalents. Thus, methyl iodide, methyl bromide, methyl chloride, methyl trifluoromethanesulfonate or methyl p-toluenesulfonate are all synthetic equivalents of the "methyl electrophile" synthon. Synthetic equivalents of acyl carbanions and α-keto carbocations will be discussed on page 17. Usually a synthetic tree of synthons is generated and possible synthetic equivalents are noted, but the final choice of a suitable synthetic equivalent for each synthon is often determined by experiment during the execution of the synthesis.
Boundary Conditions
Logic centered retrosynthetic analysis generating a tree of synthons7 is the basis of computer-assisted synthetic analysis.8 Even with the help of a computer, however, an indiscriminately exhaustive analysis would be impossibly cumbersome and of little use because much effort would be expended exploring pathways that have little or no likelihood of being useful. Rather, it is desirable to identify dislocations that are unlikely to be fruitful and abandon them, to prune the synthetic tree as it grows. The goals of reterosynthetic analysis are: (1) the identification of readily available starting materials and (2) an efficient pathway for their conversion into the synthetic target. Since the starting materials will usually have simple structures, dislocations that reduce molecular complexity are likely to lead to them. This recommends seven boundary conditions for the selection of desirable dislocations. Thus, a desirable dislocation (or transform) must (i) reduce internal connectivity by scission of rings, (ii) reduce molecular size by disconnection of chains or appendages, (iii) remove functionality, and/or (iv) simplify stereochemistry, for example, by removal of asymmetric centers. The synthetic pathways that emerge under the guidance of these boundary conditions will rapidly generate the requisite molecular complexity of the target and will, thus, involve a minimum number of steps. However, some dislocations that (v) increase molecular complexity may also be desirable if they facilitate a simplifying dislocation. For example, increasing the molecular complexity of 11 by adding unsaturation suggests a subtarget 12 that should be readily available by a 2π + 4π cycloaddition that generates two C-C bonds in a single step from the readily available starting materials 13 and 14. If the C=C bond in the subtarget 12 can be selectively hydrogenated in the presence of a C=O bond, then the final target 11 could be obtained from 12. Note that a hypothetical synthesis will be designated with dashed arrows in this book.

Another example of a potentially desirable dislocation that increases molecular complexity is provided by a strategy for preparing glyceraldehyde ketal 18. Thus, a dimeric subtarget 19 should provide 18 by oxidative cleavage of the vicinal diol functional array. Although 19 is structurally and functionally more complex than 18, it would be an excellent precursor if a method can be found to selectively ketalize the terminal vicinal diol arrays in D-mannitol, because the hexitol starting material is an inexpensive naturally derived product.

Another boundary condition is suggested by the need to (vi) avoid undesirable side reactions during the early stages of the total synthesis of a functionally complex target. Thus, side reactions are less likely if a sensitive functional array in the target is generated near the end of the synthesis. Reterosynthetically, this means that dislocations that modify (e. g. hide) or remove sites of unusually high chemical reactivity or instability are especially desirable. For example, the δ-hydroxy- β,γ-unsaturated aldehyde functional array in 20 is especially prone toward dehydration to give the dieneal 21. The aldehyde functional group can promote the dehydration. Therefore, generation of the aldehyde group in the last step of the synthesis is recommended. One possibility is to use a vicinal diol functional array in a subtarget 22 as a hidden aldehyde. Of course, the success of this strategy depends upon the feasibility of achieving oxidative cleavage of the structurally and functionally more complex subtarget 22 under suitably mild reaction conditions. This may be considered a risky strategy because the entire scheme would fail if the last step cannot be accomplished. However, the stability of the target to reaction conditions that are required to oxidatively cleave vicinal diols can be tested. However, other potential pitfalls can probably only be tested on the subtarget 22 itself. For example, will 22 tend to undergo intramolecular ketalization that cannot be easily and cleanly reversed? On the other hand, the benefits of finding a method of achieving the 22 to 20 conversion justify attempting the synthesis through this subtarget.

The ultimate goal of synthetic planning is to devise the most economical synthesis of the target. The suitability of a particular strategy must inevitably depend upon the state of the art (science?). As the availability of starting materials or methods (new or more effective) for uniting and manipulating them vary, so will the relative merits of different pathways. Put another way, a poor synthesis can become the method of choice if a method for improving a bad step can be discovered. Even a "logic centered" approach cannot produce absolute answers. What it can do is systematically generate a large number of alternative strategies for consideration in light of existing chemical knowledge.
Another concept that can guide the fruitful growth of a synthetic tree is the identification of target characteristics that direct special attention to a particular synthetic method or starting material and, thus, channel the choice of dislocations. For example, a six-membered ring invites consideration of a Diels-Alder cycloaddition as we have seen above in a strategy for the synthesis of 11 from 13 plus 14. Similarly, because of the stability associated with aromaticity, the presence of an aromatic ring in a synthetic target recommends consideration of aromatic precursors because: (1) their stability may prevent undesirable side reactions and (2) a great variety of aromatic compounds are readily available.
The facts that: (1) chemical reactions are the means of achieving skeletal construction, and (2) functionality facilitates chemical reactions, recommends a boundary condition that favors synthetic strategies that make (vii) maximum use of target-related functionality in precursors to pro- mote skeletal construction. "Target-related" refers to functionality in precursors that may be identical with or closely related to functionality in the final synthetic target.
The imposition of boundary conditions during the generation of a synthetic tree may eliminate the need for considering a large fraction of potential synthetic pathways. For example, in devising a synthesis of prostaglandin \(F_2\alpha\) we would disfavor all pathways involving a final connection between any of the carbons 16-20 which constitute an n-pentyl group. This group of atoms is an unreactive nonfunctionalized moiety. Joining two synthons at any of these bonds would require extensive functional manipulation with no obvious justification. Such strategies do not make maximum utilization of functionality.

Direct Associative Strategies
Syntheses of some target molecules or subtargets do not require the logic centered rigorous analytical approach since the molecules may be recognized as arising from the union of a number of readily available undisguised subunits which can be brought together in the proper way using standard reactions. This is known as a direct associative approach to synthetic planning. Thus, for example, strategies for the synthesis of polypeptides, almost without exception, involve the union of amino acids or suitable derivatives by the creation of amide bonds.
Generally, synthetic planning for complex molecules is intermediate between a direct associative and a logic centered approach. The initial recognition process may use logic centered analysis until a number of potentially readily available subunits or key intermediates become apparent. The choice of a particular key intermediate as a starting point channels and simplifies the analysis. This is followed by careful, usually logic centered analysis of detailed sequences that lead to the desired subunits and from them to the synthetic target.
Thus, the practical goal of logic centered analysis is to reduce a complex molecule to a set of "readily available" or "recognizable" synthons. That is, to simplify the synthetic objective to the extent that a direct associative approach becomes feasible. A knowledge of what is readily available need not precede the logic centered simplification of the problem. The simplified structures generated by such analysis may, on the contrary, become the subject of a thorough search of the chemical literature. This search might begin with a computer database such as Chemical Abstracts Online or one of the following general references:
(a) H.O. House, "Modern Synthetic Reactions", 2nd ed, Benjamin, 1972.
(b) R.B. Wagner and H.D. Zook, "Synthetic Organic Chemistry", Wiley, 1953.
(c) C.A. Buehler and D.E. Pearson, "Survey of Organic Syntheses", Wiley, 1970.
(d) A.I. Vogel, "Practical Organic Chemistry", 3red ed, Wiley, 1956.
(e) I.T. Harrison and S. Harrison, "Compendium of Organic Sythetic Methods", Wiley, 1971.
(f) L.F. Fierser and M. Fierser, "Reagents for Organic Synthesis", Wiley.
(g) "Organic Reactions", Wiley.
(h) "Organic Syntheses", Wiley.
(i) "Newer Methods of Preparative Organic Chemistry", Academic Press.
Information gleaned from the literature on established synthetic approaches to similar structures may then be used to generate further refinements of the synthetic plan by logic centered analysis. This general procedure for synthetic planning is an interactive approach. Furthermore, synthetic planning generally does not end when work in the laboratory begins. Information on molecular reactivity gleaned in the laboratory may be exploited to modify or generate new strategies. The interactions between modes of analysis and sources of relevant information are summarized below.



