11.4: Enantioselective Oxidations
- Page ID
- 168840
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Biocatalysts are also turned to be useful for asymmetric oxidations. A wide range of asymmetric oxidations using biocatalytic systems has been explored.
Baeyer-Villiger Oxidation
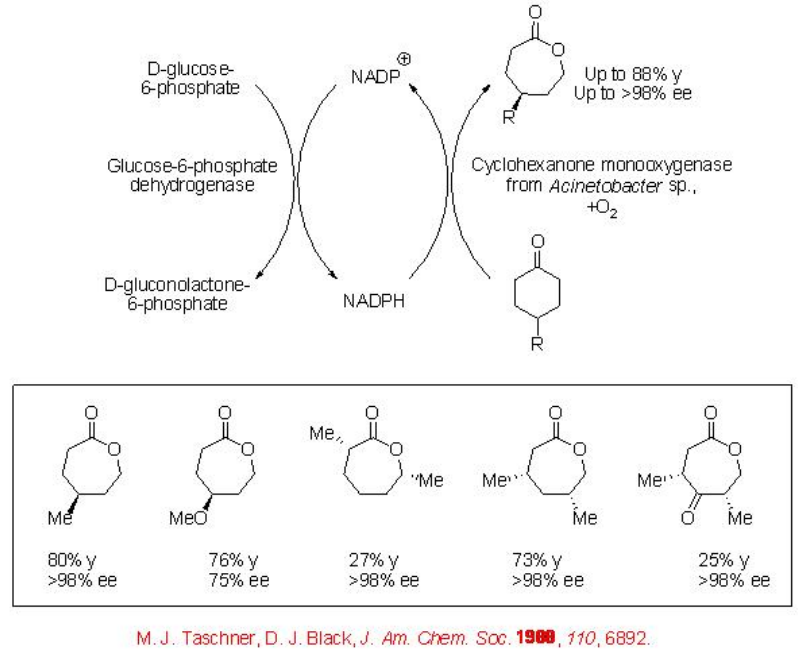
Baeyer-Villiger reaction is known for more than 100 years. However, the asymmetric version of this reaction remains as challenge for organic chemists. Depending on the nature of ketones the reaction can be carried out as a resolution of racemic ketones as well as an asymmetric desymmetrization reaction from prochiral ketones. The enzymes used for this reaction is known
as Baeyer-Villiger monooxygenases. These enzymes are cofactor dependant and are generally obtained from microbial sources. For example, 4-substituted monocyclic cyclohexanones can be oxidized into the lactones in good yield and with high enantioselectivities (Scheme \(\PageIndex{1}\)). In this process, the reduced form of the cofactor (NADPH) is needed under the formation of NADP+ that is in situ recycled using an enzymatic coupled cofactor reproduction.
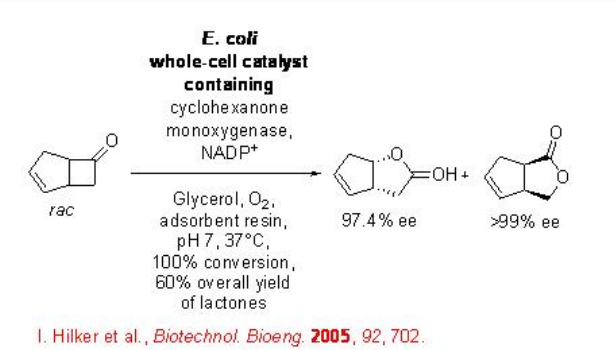
The scale up of the process has also been explored. For example, the racemic bicyclo[3.2.0]hept-2-enone with input of 25g/L proceeds oxidation in the presence of a recombinant whole-cell biocatalyst to afford regioisomeric lactones with high enantioselectivity (Scheme \(\PageIndex{2}\)).
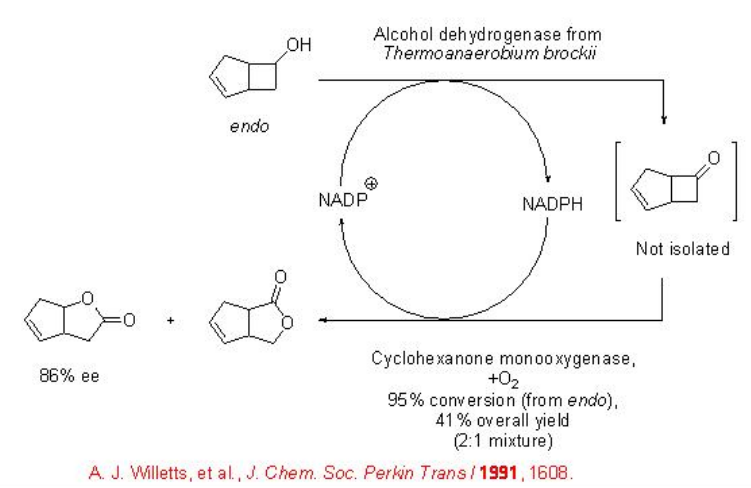
A further process improvement is the coupling of a cyclohexanone monooxygenase with an ADH from T. brockii , a cosubstrate-free “double oxidation” of an alcohol into lactones (Scheme \(\PageIndex{3}\)). In this system, the oxidized form of the cofactor (NADP+) is consumed in the initial ADH-catalyzed step, while the reduced form of the cofactor (NADPH) is then needed for the second, monooxygenase-catalyzed oxidation step. In the second step, the oxidized form of the cofactor (NADP+), which is then needed for the first step, is produced again.
Epoxidation
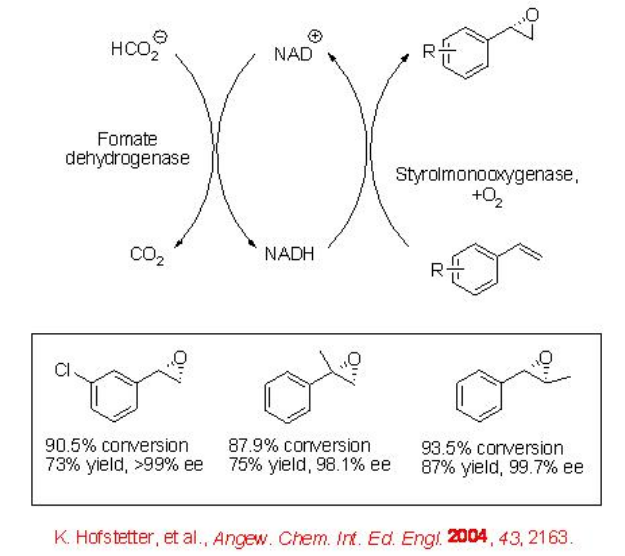
Optically active epoxides serve as versatile building blocks in organic synthesis. Besides metal and organocatalysts, cofactor dependent monooxygenase turned out to be valuable catalyst for the epoxidation of alkenes. For example, the epoxidation of styrene has been shown using a stable recombinant FAD/NADH-dependent styrene monooxygenase in aqueous-organic emulsions (Scheme \(\PageIndex{4}\)). The reaction condition is also effective for the oxidation of other styrene derivatives.
Oxidation of Amino Acids
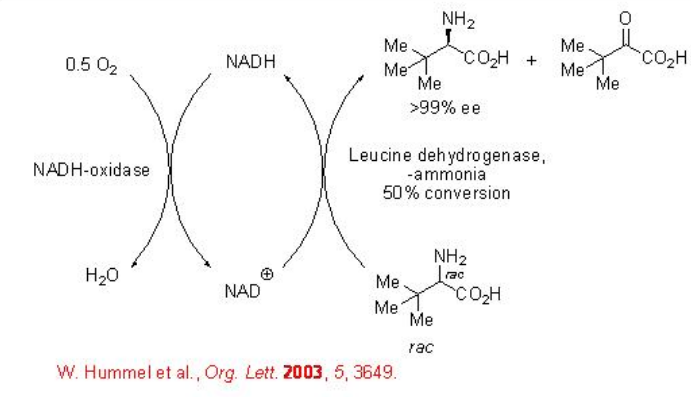
The asymmetric oxidation of amine group in amino acids provides effective method for the synthesis unnatural amino acid which is important in drug synthesis. For example, racemic tert -leucine can be oxidized to D- tert -leucine using a leucine amino dehydrogenase and an NADH-oxidase from E-coli with excellent enantioselectivity (Scheme \(\PageIndex{5}\)).
Oxidation of Alcohols
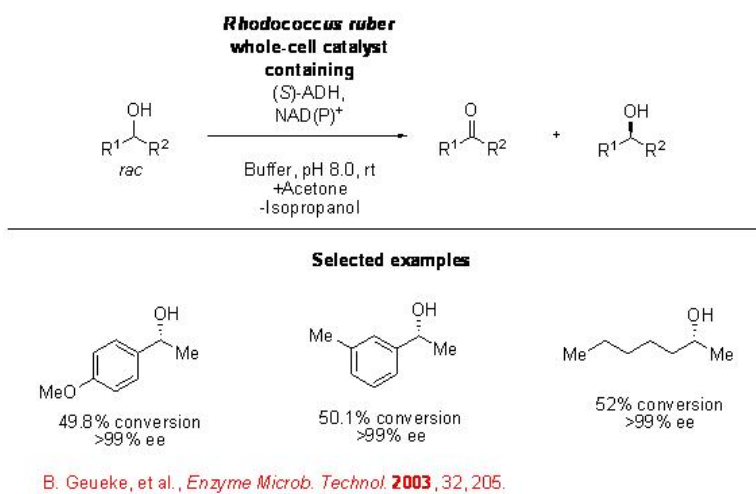
The oxidation of secondary alcohols into ketones has also been investigated using biocatalytic systems. For example, the oxidation of racemic secondary alcohols proceeds in the presence of an ADH from R. ruber (Scheme \(\PageIndex{6}\)). The recycling of the cofactor NADPH is carried out in situ using acetone, which is reduced into 2-propanol under the formation of NADP+.
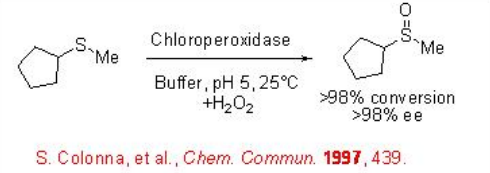
Sulfoxidation
Optically active sulfoxides play important role in organic synthesis as chiral auxiliary as well as intermediates for the construction of optically active molecules. Optically active sulfoxide is also present as structural unit in many biologically active compounds. The enzymatic oxidation of sulfides provides an effective method for the synthesis optically active sulfoxides. For example, cyclopentyl methyl sulfide undergoes oxidation in the presence of chloroperoxidase with excellent conversion and enantioselectivity.