7.4: Reactions in Ionic Liquids (IL)
- Page ID
- 168812
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Ionic liquids are composed of ions having melting points below 100°C. They are nonvolatile and facilitate the recovery and recyclability of the catalysts. Scheme \(\PageIndex{1}\) presents some the typical ILs.

7.4.1 Hydrogenation of Alkenes
Asymmetric hydrogenation of alkenes using molecular hydrogen as hydrogen source is one of the useful chemical transformation. For example, the chiral rhodium complex Rh L-1 catalyses the hydrogenation of α -acetoamide cinnamic acid and related enamides with high enantioselectivity in IL [C4C2 im][PF6 ]. The catalyst can be reused and IL can suppress the catalyst aging in some cases.

The modified rhodium complex bearing chiral diphosphine with imidazolium moieties has been used as effective catalyst for hydrogen reaction in IL (Scheme \(\PageIndex{3}\)). The catalyst can be recovered and recycled without loss of activity and selectivity.

The asymmetric hydrogenation of methyl acetamidiacrylate can be accomplished in biphasic cosolvent/IL combination in the presence of chiral rhodium complex bearing Josephose with imidazolium tag in tert -butyl methyl ether/[bmim]BF4 (Scheme \(\PageIndex{4}\)). The presence of imidazolium tag in the Josephose ligand enhances the affinity of the Rh complex for the IL and suppresses the catalyst leaching. The catalyst can be recycled without loss of activity.

The hydrogenation of tiglic acid has been successible using Ru-BINAP in [bimm]PF6 /H2O with good enantioselectivity (Scheme \(\PageIndex{5}\)). The enantioselectivity depends on the pressure of the reaction. At high pressure the presence of water increases the enantioselectivity, but low pressure show no effect.

7.4.2 Diels-Alder Reaction
Copper(II) bisoxazoline complex having imidazolium tag can catalyze the Diels-Alder reaction of N -crotonyloxazolidinones with cyclopentadienes in [C4C1 im][NTf2] (Scheme \(\PageIndex{6}\)). The catalyst can be recovered and recycled without loss of activity and enantioselectivity at least 10 times. The presence of imidazolium tag to bisoxazoline considerably enhances the recovery and reuse of the catalyst from the IL.

7.4.3 Epoxidation
The epoxidation of alkenes using chiral Mn(III) salen has been successful in a mixture of [C4C1 im][PF6]/CH2Cl2 (Scheme \(\PageIndex{7}\)). Since IL is solidified at 0°C, the reaction requires CH2Cl2 to form homogeneous solution. The catalyst and IL can be recycled with slight drop in the enantioselectivity.

The ring opening of epoxides with TMSN3 can be pursued using chiral Cr(III)salen complex in [C4C1im][OTf] and [C4C1 im][PF6] at ambient temperature (Scheme \(\PageIndex{8}\)). The catalyst can be recycled up to five times without loss of activity.
7.4.4 Epoxide Opening
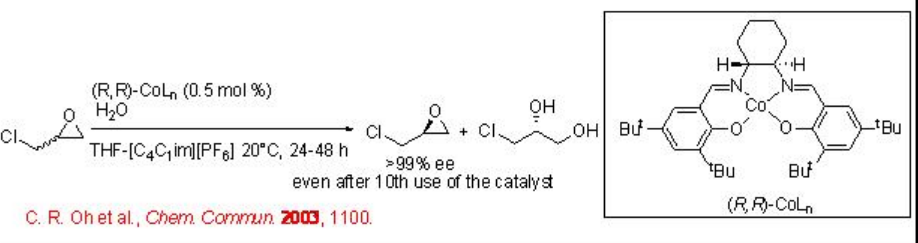
Hydrolytic resolution of racemic epoxides is effective using chiral Co(II)salen complex in THF and [C4C1 im][PF6] with excellent enantioselectivity (Scheme \(\PageIndex{9}\)). In this reaction, Co(II) is oxidized to Co(III) catalyzes the reaction. The catalyst can be recycled 10 times without loss of activity and selectivity.

7.4.5 Dihydroxylation Reaction
The asymmetric dihydroxylation of trans -stilbene has been done using OsO4 (1.5 mol%) and L-3 (2 mol%) in the presence of N -methylmorpholine N -oxide (NMO) (2.6 mol%) and [C4 C1 im][PF6] (2 mL) in acetone-water (v/v, 10/1) at °C. The catalyst can be recovered in IL and recycled up to three times without significant loss of activity and with a small amount of OsO4 leaching from the IL to organic phase.

7.4.6 Fluorination
Fluorination of β -ketoester can be accomplished employing chiral Pd-BINAP in [C4 C1 im][BF4]. (Scheme \(\PageIndex{10}\)). The reaction proceeds smoothly with good enantioselectivity and the catalyst can be recycled up to 10 times without slight loss of activity.



