7.2: Reactions in Fluorous Solvents
- Page ID
- 168810
Fluorous solvents having suitable boiling and melting points can be used as solvent. Importantly, the fluorous solvents are different from the corresponding hydrocarbons and form two layers with conventional organic solvents. Thus, some catalysts can be immobilized in fluorous solvents in biphase system and can be recovered and recycled. In addition, in some combination, the fluorous and organic solvents on heating are miscible at elevated temperature leading to a homogeneous mixture, which, after the reaction, on cooling to room temperature lead to the formation of a biphase system. The products stay in organic phase and the catalysts move to fluorous phase that can be recovered and recycled.
7.2.1 Cyclopropanation
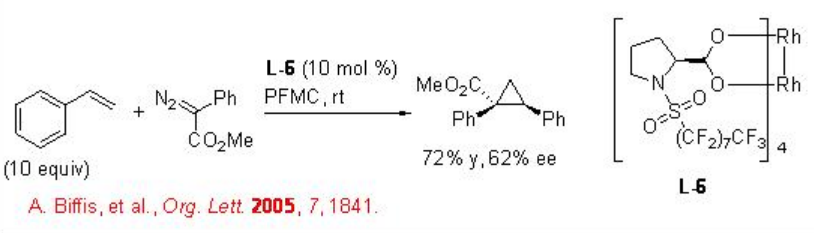
Fluorous complex tetrakis-dirhodium(II)-( S )-N-(n-perfluorooctylsulfonyl) pollinate L-6 exhibits good chemo- and diastereoselectivity in cyclopropanation of styrene (Scheme \(\PageIndex{1}\)). The advantage of the protocol is that the catalyst can be separated from the reaction mixture and recycled.