5.5: Baeyer-Villiger Oxidation (BVO)
- Page ID
- 168798
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Insertion of oxygen atom in between the ketone carbonyl and an adjacent carbon yielding the expanded ester is called as Baeyer-Villiger oxidation (BVO). Under the influence of a chiral reagent, this oxidation can be carried out asymmetrically. In case of a racemic ketone, a chiral catalyst has the potential of performing a kinetic resolution. A century after its discovery, the catalytic asymmetric BVO remains as one of the most powerful methods to convert a ketone into an ester proceeding by insertion of an oxygen atom into a bond.
Metal-Catalyzed Reactions
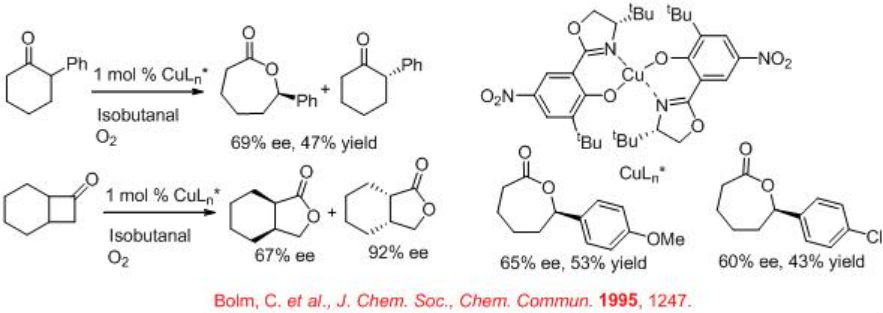
Copper(II) complexes with oxazoline-based ligands are studied for the oxidation of substituted cyclic ketones to give lactones with high enantioselectivity (Scheme \(\PageIndex{1}\)). These reactions employ isobutanal as co-reductant under aerobic conditions. During the reaction isobutanal is oxidized to the corresponding carboxylic acid.
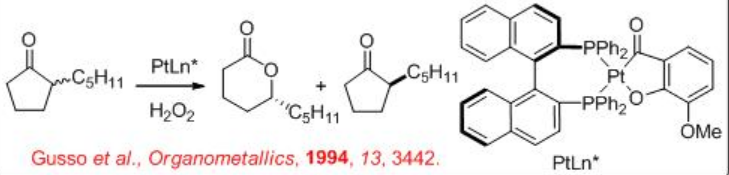
Platinum complexes bearing chiral phosphines catalyze oxidation of substituted cyclic ketones in the presence of hydrogen peroxide (Scheme \(\PageIndex{2}\)). Coordination of Pt and peroxide to the carbonyl leads to the formation of a metallocycle that could be decomposed into the target lactone. Chiral ligands associated with Pt allow for diastereomeric transition states, which discriminate between the two possible migrating carbon atoms resulting in enantioselectivity.
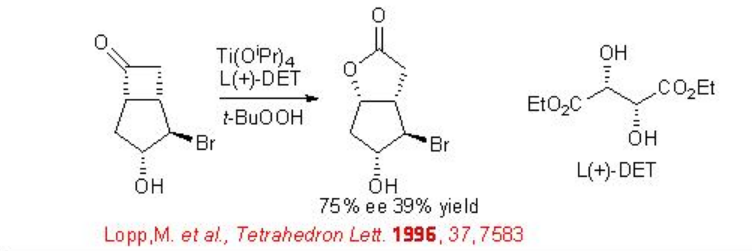
The reaction conditions used for the enantioselective epoxidation of allylic alcohols (Sharpless epoxidation) is also effective for the oxidation of substituted cyclobutanones to give lactones with moderate to good enantioselectivity (Scheme \(\PageIndex{3}\)).
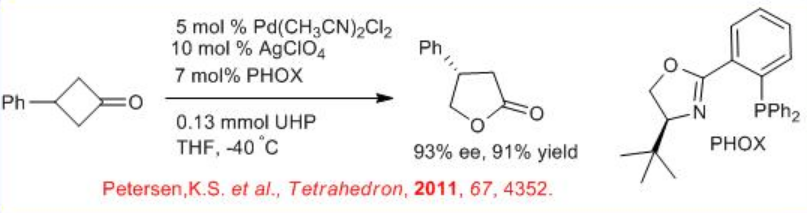
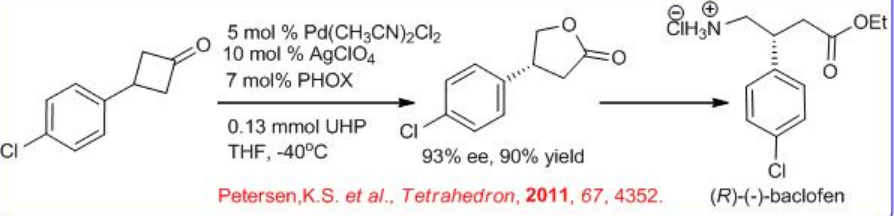
The oxidation of symmetrical cyclobutanones is effective using chiral palladium complex bearing phosphinooxazoline ( PHOX) in the presence of urea hydrogen peroxide. For example, prochiral 3-substituted cyclobutanones undergoes oxidation to give γ -lactones, which can be recrystallized to obtain the target products with 93% ee and 91% yield. This procedure has been utilized for the synthesis of GABA-B receptor agonist (R) -( _ )-baclofen (Scheme \(\PageIndex{5}\)). The racemic form of baclofen is commercially available to treat spasticity and alcoholism; however, the ( R ) - isomer has been shown to be predominantly responsible for the molecule's bioactivity. The molecule has been the target of many asymmetric syntheses. Several of these strategies start from enantioenriched lactone using enzymatic BVO or from an enantioselective C-H insertion.
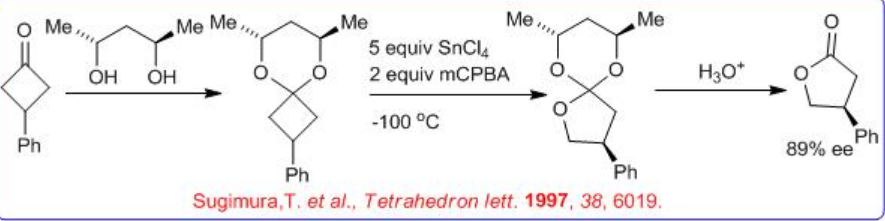
In addition to the metal-catalyzed BVOs, chiral auxiliary approach is also followed to synthesis lactone with good enantioselectivity (Scheme \(\PageIndex{6}\)). For example, reaction of optically active 1,3-diol with an achiral cyclobutanone can give chiral ketal. The latter can be reacted with m CPBA and SnCl4 to give an orthoester, which upon acidic work-up affords the lactone.
Enzyme Catalyzed Reactions
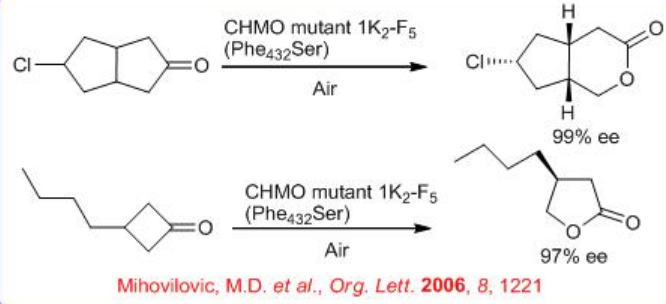
Baeyer-Villiger monooxygenases are enzymes that catalyze the insertion of an oxygen atom in a ketone, next to the carbonyl carbon atom. So far, only a limited number of BVMO have been identified from bacteria and fungi. These enzymes typically contain FAD or FMN as a cofactor and catalyze highly regio- and stereoselective oxygenations at the expense of NAD(P)H and molecular oxygen. Bio-catalyzed BVO proceeds with high levels of enantioselectivity. For example, cyclohexanone monooxygenase (CHMO), a bacterial flavoenzyme, carries out an oxygen insertion reaction on cyclohexanone to form a seven-membered cyclic product, ε-caprolactone (Scheme \(\PageIndex{7}\)). This reaction involves the four-electron reduction of O2 at the expense of a two-electron oxidation of NADPH and a two-electron oxidation of cyclohexanone to form ε-caprolactone. The CHMO has been employed successfully for the oxidative desymmetrization of cyclobutanone and cyclopentanone rings with high enantioselectivity. CHMO mutant 1K2 -F5 (Phe432 Ser) has been used with air as the oxidant in a whole-cell process. Mutant Phe432 Ser also tested for oxidative desymmetrization of a set of 4-substituted cyclohexanone derivatives (methyl, ethyl, methoxy, chloro, bromo, iodo) and in all cases enantioselective transformations are observed with up to 99% ee.
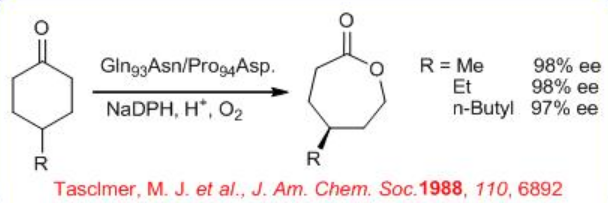
Similarly, PAMO mutant Gln93 Asn/ Pro94 Asp is tested for the asymmetric desymmetrization of 4-substituted cyclohexanone derivatives to give chiral lactones with high enantioselectivity (Scheme \(\PageIndex{8}\)). It is interesting to note that the absolute configuration of the lactone products is opposite to what is observed with the thermolabile cyclohexanone monooxygenase (CHMO) as the catalyst.
Reactions using Organocatalysis
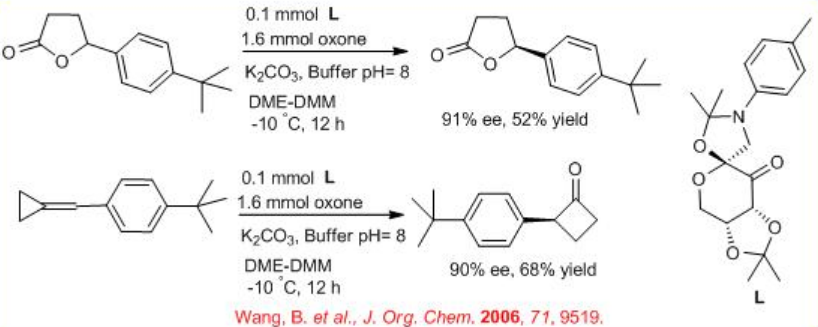
Readily available glucose-derived oxazolidinone containing ketone can be employed for BVO of a variety of benzylidenecyclopropanes in the presence of oxone (Scheme \(\PageIndex{9}\)). Optically active α -aryl- γ-butyrolactones and α -aryl- γ - methyl- γ-butyrolactones can be obtained in reasonable yields and enantioselectivities. The reaction works via in situ epoxide rearrangement and BVO. Chiral cyclobutanones can also be obtained by suppressing BVO with more ketone catalyst and less oxone.