5.4: Enantioselective Sulfoxidation
- Page ID
- 168797
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Enantiopure sulfoxides serve as chiral auxiliary as well as intermediates for the synthesis of optically active compounds. Optically active sulfoxide structural unit is also present in many compounds that exhibit interesting biological properties (Scheme \(\PageIndex{1}\)). Development of methods for the asymmetric sulfoxidation has thus been active topic in asymmetric catalysis. This lecture covers the common methods that are used for the synthesis of optically active sulfoxides.

5.4.1 Enzyme-Catalyzed Reactions
Enzyme catalyzed asymmetric oxidation of sulfides provides effective methods for the synthesis of optically active sulfoxides. For example, cyclohexanone monooxygenase (CHMO), a bacterial flavoenzyme, catalyzes the oxidation of prochiral thioethers with excellent enantioselectivity (Scheme \(\PageIndex{2}\)).

5.4.2 Chiral Reagents Based Reactions
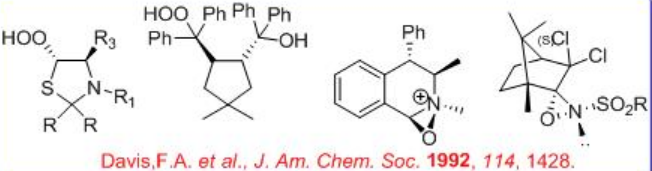
Chiral reagents have been used for the oxidation of prochiral sulfides. For example, chiral hydroperoxides, N -sulfonyl oxaziridines and chiral oxaziridines can oxidize prochiral sulfides to optically active sulfoxides with moderate to good enantioselectivity (Scheme \(\PageIndex{3}\)).
In addition, chiral sulfinates are precursors of chiral sulfoxides (Scheme \(\PageIndex{4}\)). This approach is of preparative interest to provide the sulfoxides with high enantioselectivity. The important issue is need to prepare the menthyl- p -tolylsulfinates from L-(-)-menthol and then to separate them.

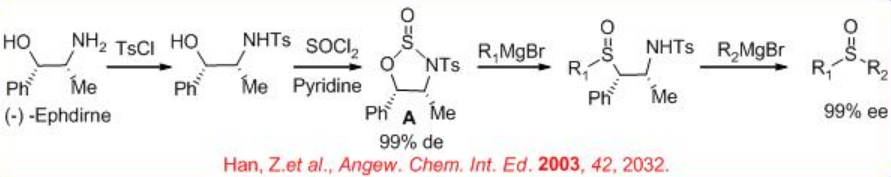
Furthermore, N -tosyl-norephedrine can be reacted with thionyl chloride to afford heterocyclic compound A, which could be reacted via the sequential addition of R1MgX and R2MgX, in a one-pot procedure to give sulfoxides in >99% ee (Scheme \(\PageIndex{5}\)). The configuration depends on the order of introduction of the two Grignard reagents.
5.4.3 Metal-Catalyzed Reactions
5.4.3.1 Reactions with Diethyl Tartrates
In the middle of 1980, Kagan and Modena groups independently modified the conditions that were employed by Sharpless group for the asymmetric epoxidation of allylic alcohols, and used for the oxidation of sulfides. The modified conditions involve the combination of Ti(OiPr)4, ( R,R)- diethyl tartrate (DET) and t -BuOOH (TBHP) in water (Scheme \(\PageIndex{6}\)). The replacement of TBHP with cumyl hydroperoxide (CHP) led to improvement in the enantioselectivity of the sulfoxide.

5.4.3.2 Reactions with Tridentate Ligands
In the middle of 1990, vanadium complexes having the tridentate Schiff base ligands L1-2 derived from optically active amino alcohols and aryl aldehydes have been studied for the oxidation of sulfides in the presence of aq. H2O2 as terminal oxidant (Scheme \(\PageIndex{7}\)). The catalysts are prepared in situ and the effect of series Schiff base ligands is studied.

In case of di- tert -butyldisulfide, monoxidation occurs selectively with up to > 90% ee (Scheme \(\PageIndex{8}\)).

Subsequently, the reaction has also been found to be effective with Fe(acac)3 in the presence of additive such as p-methoxybenzoic acid (Scheme \(\PageIndex{9}\)). For example, the oxidation of p -chlorophenyl methyl sulfide can be accomplished with 92% ee and 60% yield. In some cases, kinetic resolution is observed.

5.4.3.3 Reactions with Salen Based Ligands
Chiral Ti-salen has been found to be effective catalyst for the oxidation of sulfides in the presence of urea hydrogen peroxide (UHP) or aqueous H2O2 . First, Ti-salen is converted into cis - μ -dioxo Ti-dimer that reacts with H2O2 to give peroxo species. The latter can oxidize the sulfide to sulfoxide (Scheme \(\PageIndex{10}\)). The oxidation of several alkyl aryl sulfides can be accomplished with 92–99% ee.

Subsequently, Fe(salan) has been found to catalyze the oxidation of sulfides in the presence of a queous H2O2 in water (Scheme \(\PageIndex{11}\)). This procedure has the advantages of high catalytic turnover number (TON) of 8000 as well as the use of water as reaction medium.

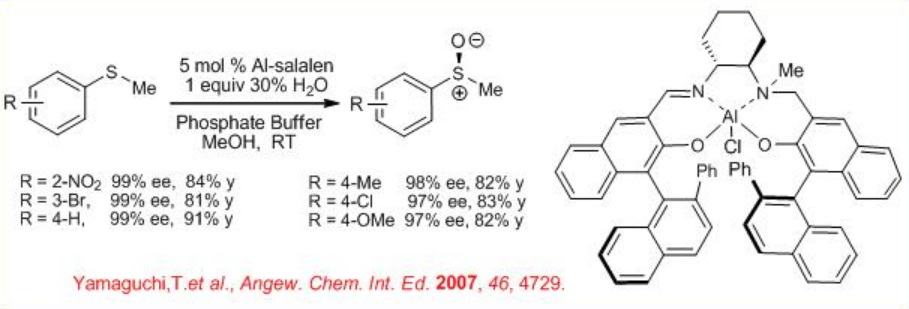
Furthermore, Al(salalen), which is compatible in water, catalyzes the oxidation of sulfides with aqueous H2O2 at room temperature in phosphate buffer condition (Scheme \(\PageIndex{12}\)). The reactions of a variety of sulfides have been demonstrated with high enantioselectivity.
Meanwhile, chiral Ru(NO)-salen has been found to catalyze the sulfoxidation under aerobic conditions in the presence of water under visible light irradiation at room temperature (Scheme \(\PageIndex{13}\)). Unlike biological oxygen atom transfer reactions that need a proton and electron transfer system, this aerobic oxygen atom transfer reaction requires neither such a system nor a sacrificial reductant.

Although the mechanism of this oxidation has not been completely clarified, some experimental results support the notion that an aqua ligand coordinated with the ruthenium ion serves as a proton transfer agent for the oxygen activation process, and it is recycled and used as the proton transfer mediator during the process.


