2.1: Enantioselective Ene and Cycloaddition Reactions
- Page ID
- 168773
Alder-ene and Diels-Alder reactions are six electron pericyclic processes between a “diene” or an alkene bearing an allylic hydrogen and an electron-deficient multiple bond to form two bonds σ with migration of the π bond. The lecture covers the examples of recent developments in enantioselective intermolecular Alder-ene glyoxylates with alkenes.
Carbonyl-Ene Reaction
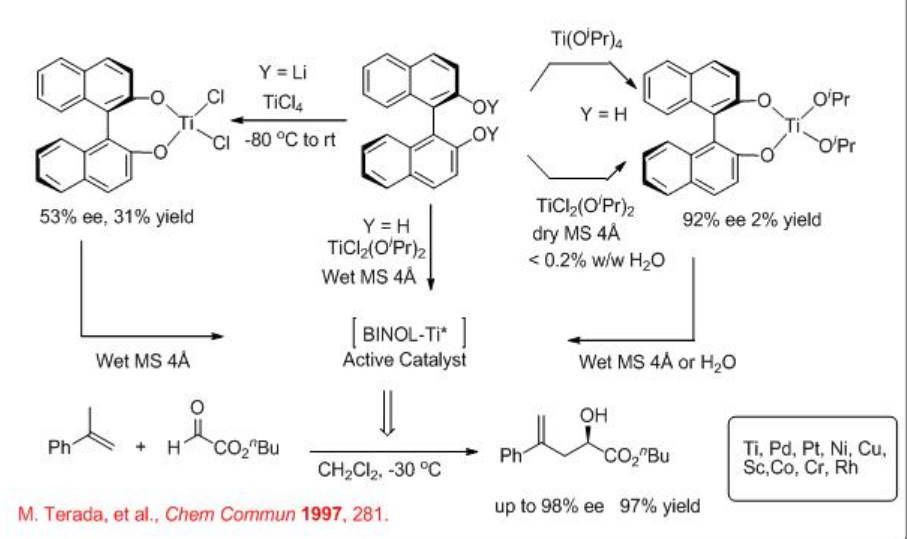
Chiral Lewis acid catalyzed enantioselective ene reaction is one of the efficient methods for atom economical carbon-carbon bond formation. For example, Ti-BINOL prepared in situ catalyzes efficiently the carbonyl-ene reaction of glyoxylate with α -methylstyrene in the presence of molecular sieves with high enantioselectivity (Scheme \(\PageIndex{1}\)).
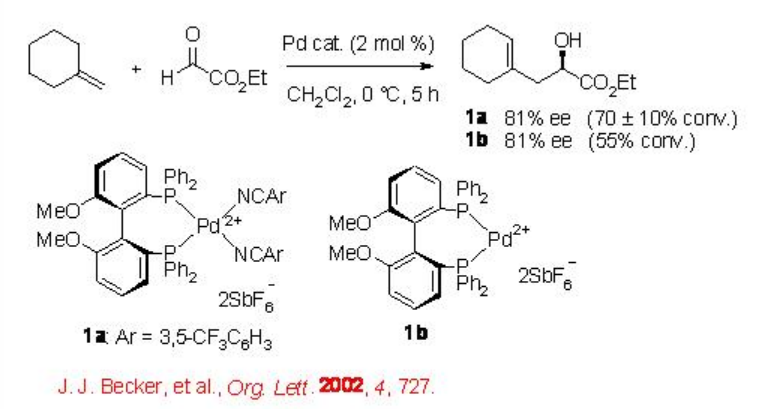
Besides the early transition metal based Lewis acid catalysts, square planar dicationic late transition metal complexes bearing C 2-symmetric diphosphine ligands have also been considerably studied as chiral Lewis acids for carbonyl-ene reactions. For example, the isolated MeO-BIPHEP-Pd complex 1a bearing electron withdrawing benzonitrile as the labile, stabilizing ligands has been used for the ene reaction of ethyl glyoxylate with up to 81% ee (Scheme \(\PageIndex{2}\)). The isolated 1a exhibits more catalytic activity compared to that 1b which is in situ generated although both offer similar enantioselectivity.
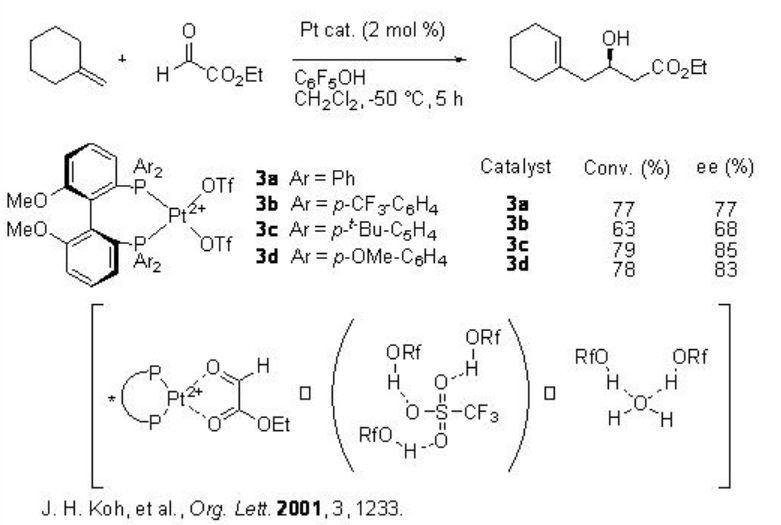
MeO-BIPHEPs-Pt complexes 3 with OTf - as counter anion also exhibit similar catalytic activity and selectivity in the asymmetric glyoxylate ene reaction (Scheme 3). The addition of phenol facilitates the reaction by trapping the OTf anion and traces of water.
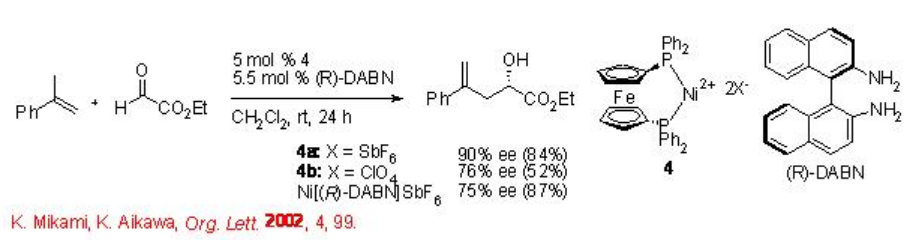
The glyoxylate ene reaction is also effective using tropox dicationic DPPF-Ni complex 4 with enantioselectivity up to 90% ee (Scheme \(\PageIndex{4}\)).
The glyoxylate-ene reaction can also be carried out using chiral C 2-symmetric bisoxazolinyl copper(II) complexes 5 and 6 as Lewis acid catalysts (Scheme \(\PageIndex{5}\)). The aqua complex is air and water stable and exhibits only slight decrease in the reaction rate compared to the anhydrous complex 6 . The sense of asymmetric induction depends on the oxazoline ring substituents, which can be rationalized by the tetrahedral and square-planer intermediates to account for the absolute configuration of the products.
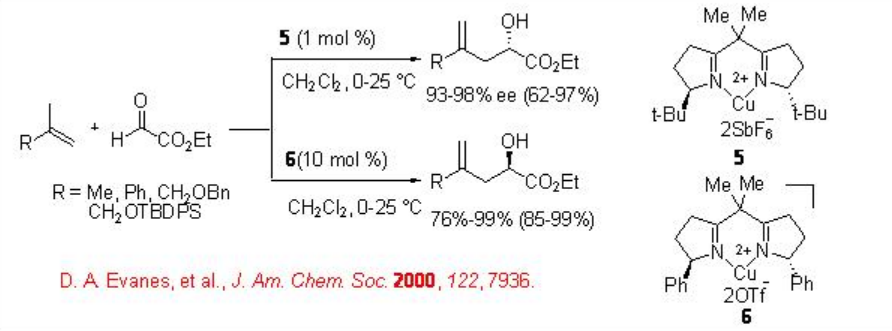
In addition, chiral C2-symmetric trivalent pybox-Sc complex 7 is studied for the carbonyl-ene reactions with N -phenyl glyoxamides (Scheme \(\PageIndex{6}\)). The ene products are obtained with excellent diastereo- and enantioselectivity. Presumably, the products are formed via proton transfer from the β - cis substituent through an exo -transition state.
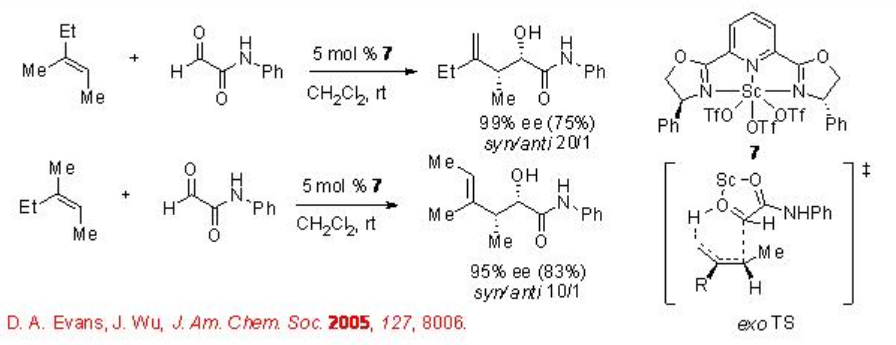
Co and Cr-based chiral complexes have also been explored for the carbonyl-ene reaction with glyoxylates. For example, chiral β-ketoiminato complex 8 catalyzes efficiently the reaction of 1,1-disubstiuted alkene and glyoxyl derivative in high enantioselectivity (Scheme \(\PageIndex{7}\)). Similar to the earlier described Pd, Pt and Ni-based catalysts, hexafluoroantimonate as a counter anion is found to be the most effective.
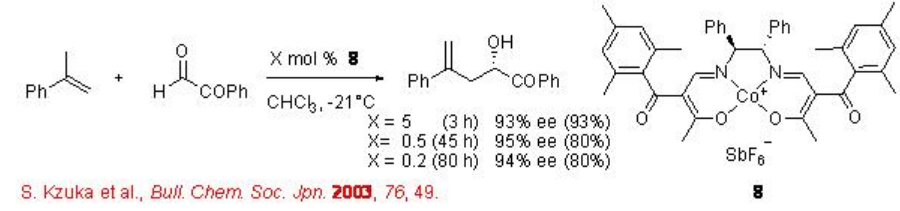
Chiral Cr(III)-salen complex 9 bearing adamantyl group in the salen ligand has been used for the reaction of ethyl glyoxylate with 1,2-disubstituted alkenes (Scheme \(\PageIndex{8}\)). The catalyst can be prepared in multigram scale and the ene products are obtained with up to 92% ee. The presence of adamantyl substituent essential for the enhancement in the enantioselectivity.
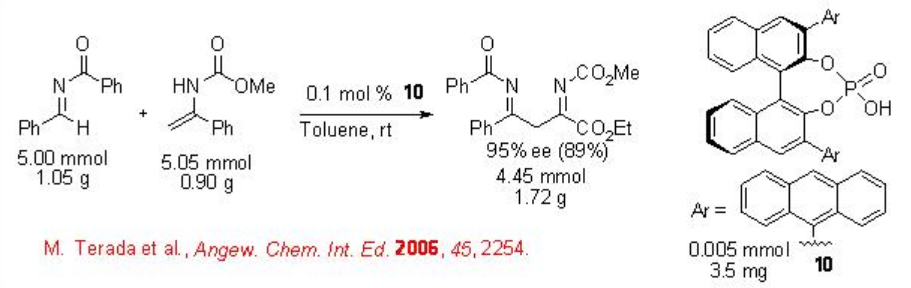
Besides the metal based catalysts, chiral organocatalysts have also been considerably explored during the recent years for the carbonyl-ene reactions. For example, the chiral phosphoric acid 10 as a chiral Bronsted acid catalyzes readily the enantioselective aza-ene reaction of enamides to imines with excellent enantioselectivity even on a gram scale (Scheme \(\PageIndex{9}\)).
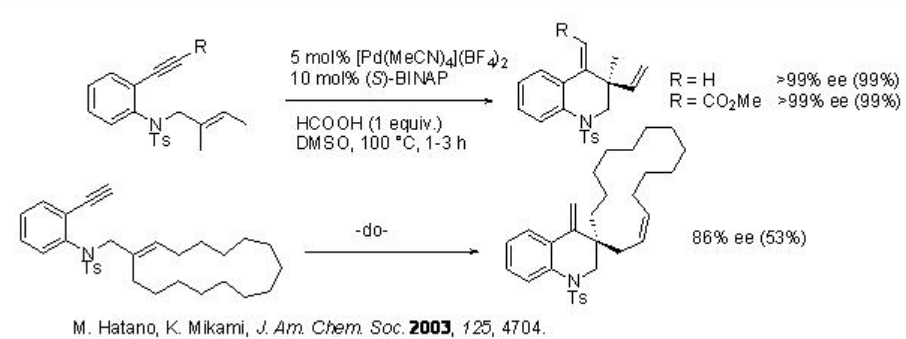
Besides the intermolecular reactions, intramolecular version of this reaction has also been well explored using chiral metal as well as chiral phosphoric acids as catalysts. For example, the palladium-phosphine complex catalyzed cyclization of 1,7-enyenes bearing benzene ring takes place efficiently to afford six membered quinoline derivatives with quaternary stereogenic centers as single enantiomer (Scheme \(\PageIndex{10}\)).
Diels-Alder Type Reactions
Asymmetric intra- and intermolecular Diels-Alder reactions have made remarkable progress using chiral metal complexes as catalysts. Subsequently, several studies are focused on the use of chiral organocatalysis for this reaction. Since the organocatalysis based reactions are covered in module I, this lecture covers recent examples of the metal catalyzed reactions.
Intramolecular [4+2]-Cycloaddition
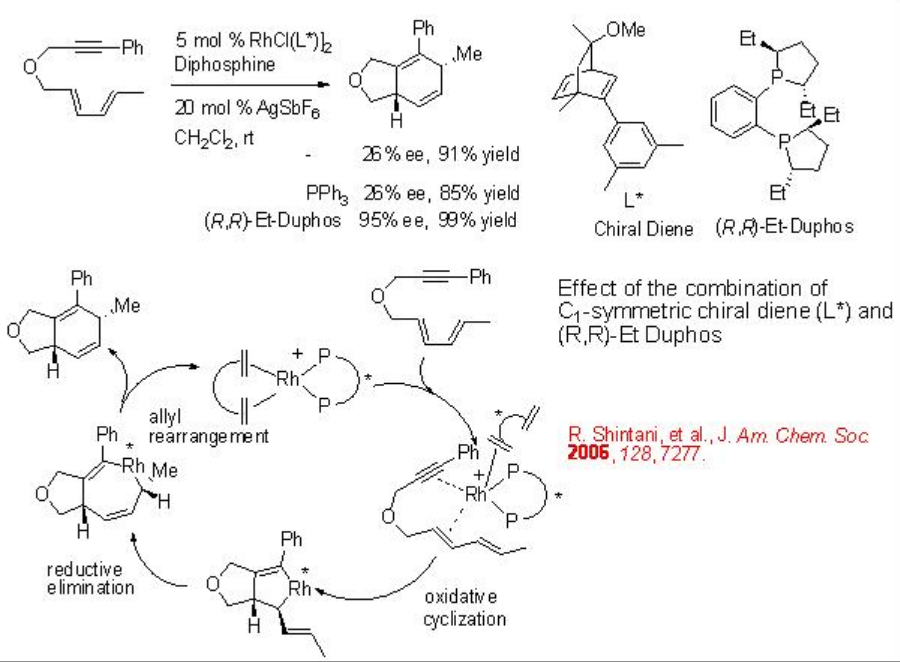
Intramolecular Diels-Alder reactions of unactivated dieneynes provide powerful tool to construct 5,6- or 6,6-fuzed rings. These fuzed rings can be inducted in the synthesis of many natural products. Therefore, a number of methods using transition metal catalysis have been developed over the past two decades. The chiral Rh complex bearing chiral diene and chiral phosphine has been shown to give better enantioselectivity compared to that bear achiral diene and chiral phosphine complex (Scheme \(\PageIndex{11}\)).
Intermolecular Diels-Alder Reactions
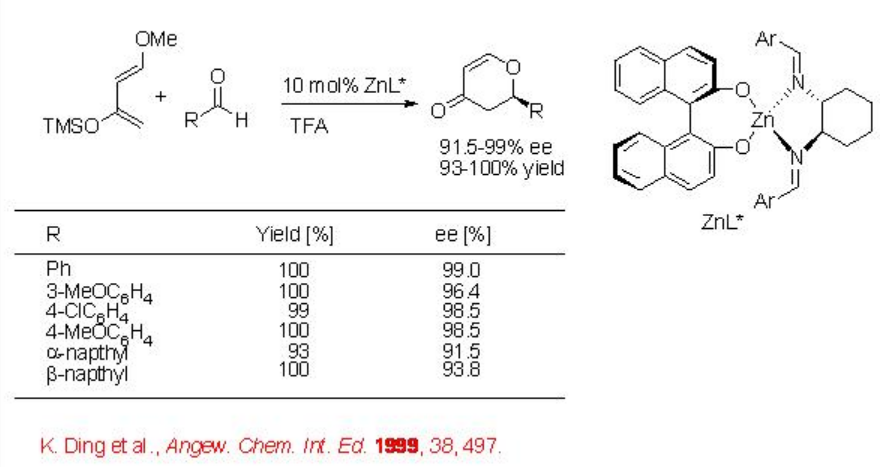
Intermolecular hetero Diels-Alder reactions have also been extensively explored using both chiral metal complexes as well as chiral organocompounds as catalysts. Since the use of chiral organocatalysis has been covered in module I, this section focuses on few examples using chiral metal complexes as the catalysts. The reaction of benzaldehyde with Danishefsky's diene proceeds in the presence of BINOL/diimine/Zn complex with excellent enantioselectivity and yield (Scheme \(\PageIndex{12}\)).
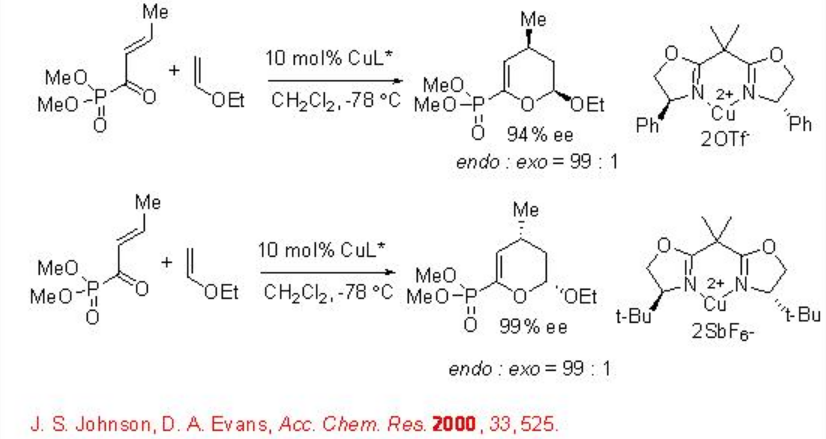
Chiral box-Cu(II) complexes are found to be excellent catalysts for a variety of hetero Diels-Alder reactions (Scheme \(\PageIndex{13}\)).
The readily accessible oxazaborolidine-aluminum bromide catalyst catalyzes the reaction of furan with diethyl fumarate with excellent enantioselectivity (Scheme \(\PageIndex{14}\)).
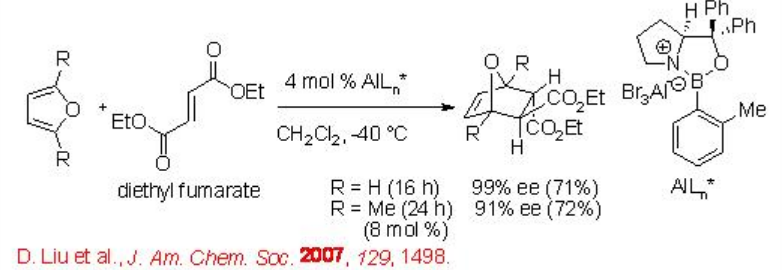
Scheme \(\PageIndex{14}\)


