16.6: Oxidative Damage to cells, Vitamin C, and Scurvy
- Page ID
- 106397
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)While the hydroxide radical can be a beneficial 'detergent' in the atmosphere, it is harmful when present in a living cell. Hydroxide radical is one of the reactive oxygen species (ROS) that we learned about in chapter 15. Recall that ROS are continuously produced as minor but harmful side-products in the reduction of \(O_2\) to \(H_2O\) in respiration.
You may recall from your general chemistry course that molecular oxygen exists in two states: 'singlet' oxygen has a double bond and no unpaired electrons, while 'triplet' oxygen has a single O-O bond and two unpaired electrons. Molecular orbital theory - and experimental evidence - show that the triplet state is lower in energy.
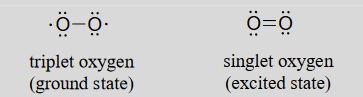
Figure 16.6.1
ROS are highly reactive oxidizing agents, capable of inflicting damage to DNA, proteins, and the lipids of cell membranes - they are thought to play a major role in the aging process. Hydroxide radical, for example, can initiate a radical chain reaction with the hydrocarbon chain of an unsaturated membrane lipid molecule, resulting in the formation of lipid hydroperoxide.
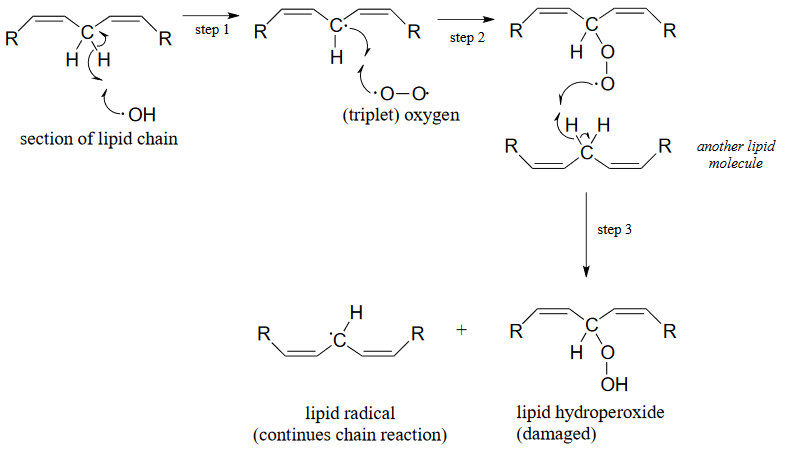
Figure 16.6.2
The allylic lipid radical formed as the result of homolytic hydrogen abstraction by hydroxide radical (step 1 above) reacts with one of the unpaired electrons in triplet oxygen (step 2) forming a peroxy radical. This radical species in turn homolytically abstracts a hydrogen from another lipid molecule (step 3), thus propagating the chain.
Many edible plants contain various antioxidant compounds, also known as 'free radical scavengers', which serve to protect cells from the oxidative effects of hydroxide radical and other harmful radical intermediates. Simply put, a free radical scavenger is a molecule that reacts with a potentially damaging free radical species, forming a more stable radical species which can be metabolized by the body before any damage is done to cell constituents.
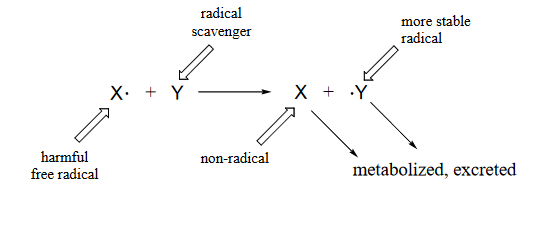
Figure 16.6.3
In the introduction to this chapter, we learned about scurvy, the disease long dreaded by sailors, and how it is caused by a deficiency of ascorbic acid (vitamin C) in the diet. We will soon get to the connection between ascorbic acid and scurvy, but first, let's look at how ascorbic acid functions as a free radical scavenger in your body.
The \(pK_a\) of ascorbic acid is about 4.1, so in a physiological environment it exists mainly as ascorbate anion, the conjugate base. When ascorbate encounters a hydroxide radical (or any other potentially damaging radical species), it donates a single electron, thus reducing the hydroxide radical to hydroxide ion and becoming itself an ascorbyl radical.
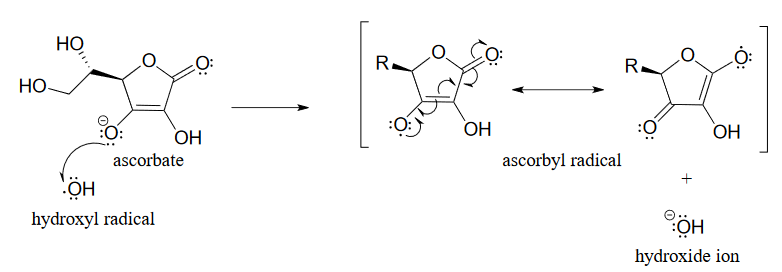
Figure 16.6.4
The ascorbyl radical is stabilized by resonance. The end result of this first step is that a very reactive, potentially harmful hydroxide radical has been 'quenched' to hydroxide ion and replaced by a much less reactive (and thus less harmful) ascorbyl radical.
The ascorbyl radical can then donate a second electron to quench a second hydroxide radical, resulting in the formation of dehydroascorbate, the oxidized form of ascorbate.
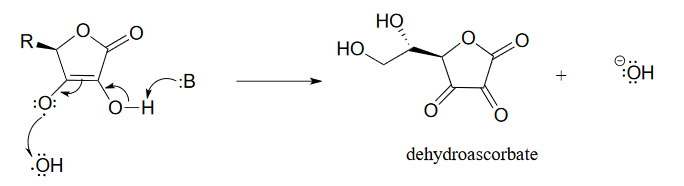
Figure 16.6.5
One ascorbate molecule is thus potentially able to scavenge two harmful radical species.
Dehydroascorbate is subsequently either broken down and excreted, or else enzymatically recycled (reduced) back to ascorbic acid. You were invited to propose a mechanism for the latter (redox) step in problem 15.10. J. Am. Coll. Nutr., 2003, 22, 18
We learned in the introduction to this chapter about the gruesome effects of long-term ascorbic acid deficiency. What, then, is the chemical connection between ascorbic acid and scurvy?
The symptoms association with scurvy are caused by the body's failure to properly synthesize collagen, the primary structural protein in our connective tissues. Essential to the stability of collagen is its ability to form a unique triple-helical structure, in which three protein strands coil around each other like a woven rope. Collagen strands are not able to pack together properly into their triple helix structure unless certain of their proline amino acid residues are hydroxylated: the electronegative OH group on hydroxyproline causes the five-membered ring in the amino acid to favor a particular 'envelope' conformation (section 3.2) as well as the 'trans' peptide conformation, both of which are necessary for stable triple-helix formation.
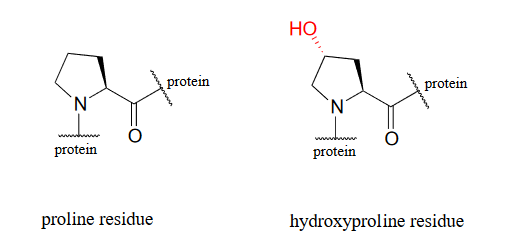
Figure 16.6.6
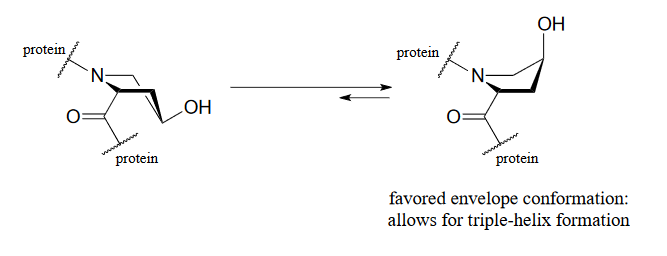
Figure 16.6.7
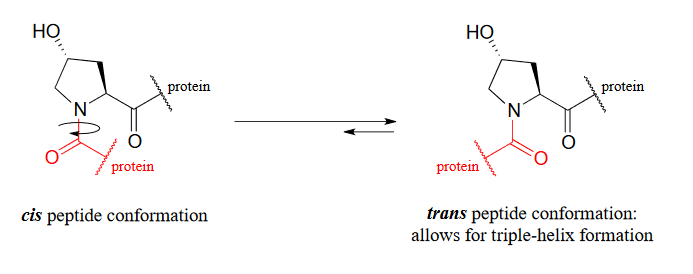
Figure 16.6.8
Proline hydroxylase, the enzyme responsible for this key modification reaction, depends in turn upon the presence of ascorbate. The hydroxylating reaction is complex, and involves electron-transfer steps with enzyme-bound iron - mechanistic details that are well outside of our scope here, but which you may learn about in a bioinorganic chemistry course. It is enough for us to know that iron starts out in the \(Fe^{+2}\) state, and during the course of the reaction it loses an electron to assume the \(Fe^{+3}\) state. In order for the enzyme to catalyze another reaction, the iron must be reduced back to its active \(Fe^{+2}\) state - it must accept a single electron. The donor of this single electron is ascorbate.
(For more information, see Crit. Rev. Biochem. Mol. Biol. 2010, 405, 106.)
So, to sum up: If we fail to get enough ascorbic acid in our diet (in other words, if we don't eat our fruits and vegetables!) the iron in our proline hydroxylase enzymes won't be returned to the active \(Fe^{+2}\) state, so the catalytic cycle is broken and we can't turn prolines into hydroxyprolines. Without the hydroxy group, the proline residues of our collagen proteins won't assume the proper conformation, and as a consequence the collagen triple helix structures will be unstable. At physiological temperature, our collagen will literally melt apart - and with it, our gums, our capillaries, and anything else held together by connective tissue. This is scurvy.
You have probably heard that many fruits and vegetables contain natural 'polyphenol' antioxidant compounds that are thought to be beneficial to our health. Apigenin, for example, is found in parsley and celery, while the skins of grapes used to produce red wine are particularly rich in resveratrol, as well as many other polyphenols. Curcumin is the compound responsible for the distinctive yellow color of turmeric, a ubiquitous spice in Indian cuisine.
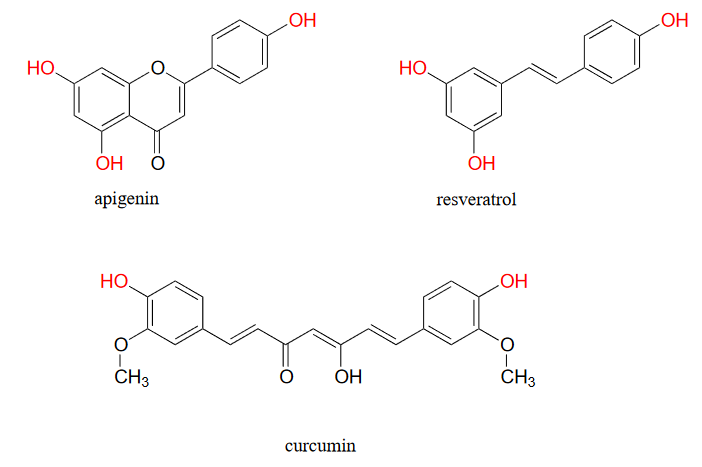
Figure 16.6.9
While much remains to be learned about exactly how these polyphenols exert their antioxidant effects, it is likely that they, like ascorbic acid, act as radical scavengers. For example, resveratrol could donate a single electron (and a proton) to hydroxide radical to reduce it to water. The phenolic radical that results is stabilized by resonance, and is much less likely than hydroxide radical to cause damage to important biomolecules in the cell.
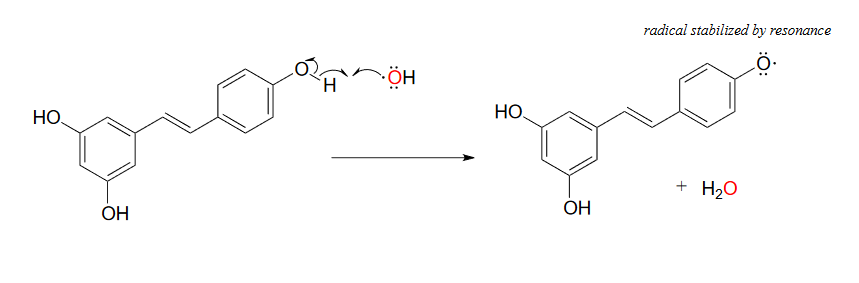
Figure 16.6.10


