12.E: Reactions at the α-Carbon, Part I (Exercises)
- Page ID
- 106369
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
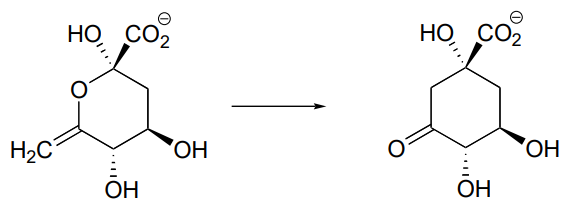
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)P12.1: The enzyme ribulose-5-phosphate isomerase (EC 5.3.1.6), which is active in both the Calvin cycle and the pentose phosphate pathway, catalyzes an aldehyde-to-ketone isomerization between two five-carbon sugars.
- Draw a mechanism for this step.
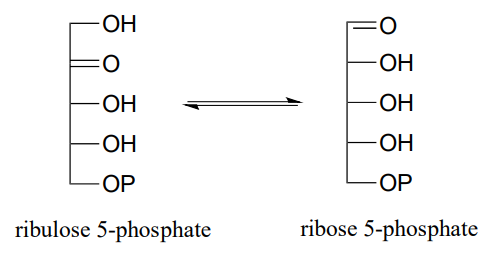
- What \(^1H-NMR\) signal would most clearly differentiate the aldose from the ketose in this reaction?
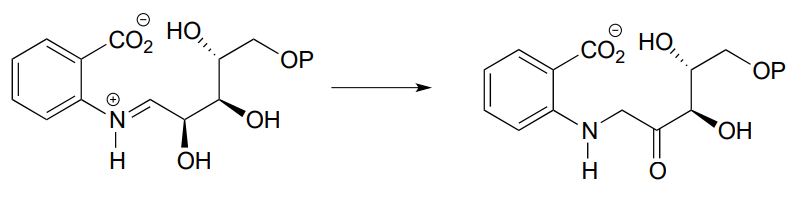
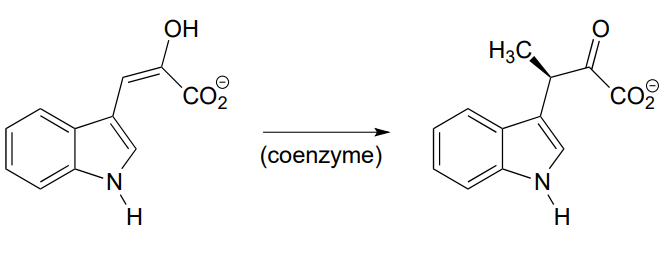
P12.2: Provide a likely mechanism for the reaction below, from tryptophan biosynthesis (EC 5.3.1.24) Hint: this is mechanistically very similar to a carbonyl isomerization reaction.
P12.3:
- Draw the product of an aldol addition reaction between pyruvate and glyoxylate (EC 4.1.3.16):
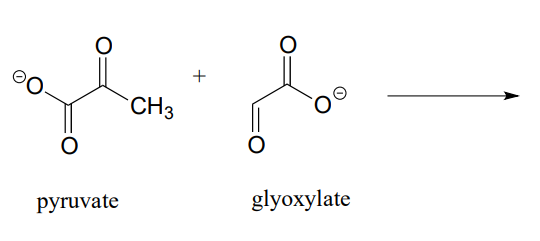
- Draw the product of an aldol addition reaction between two molecules of pyruvate (EC 4.1.3.17).
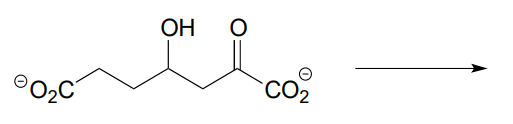
- The molecule below undergoes a retroaldol cleavage reaction in E. coli (J. Biol. Chem. 2012, 287, 36208). Draw the structure of the products.
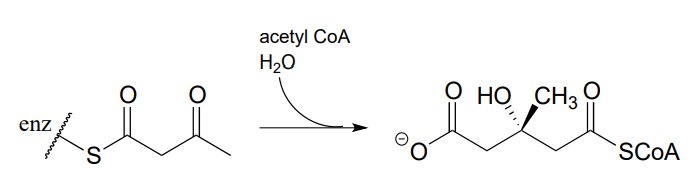
- Propose a mechanism for this early reaction in the biosynthesis of isoprenoids (EC 2.3.3.10). Hint: this is an aldol reaction, followed by thioester hydrolysis.
- The carbon-carbon bond cleaving reaction below was reported to take place in many species of bacteria. Predict the structure of product X, and draw a mechanism for the reaction. Assume that an imine linkage to an enzymatic lysine residue does not play a part in the mechanism. (J. Bacteriol. 2009, 191, 4158).
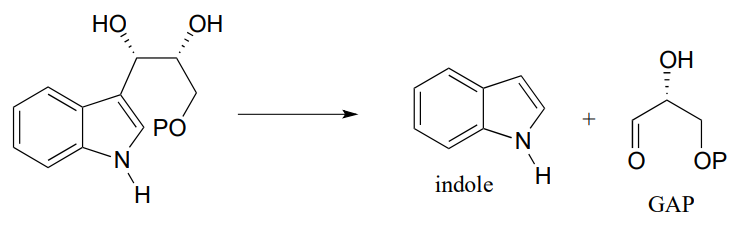
P12.4: Below is a step in the biosynthesis of tryptophan. Draw a likely mechanism. Hint: you will need to show an enamine to imine tautomerization step first, then the carbon-carbon bond breaking step will become possible.
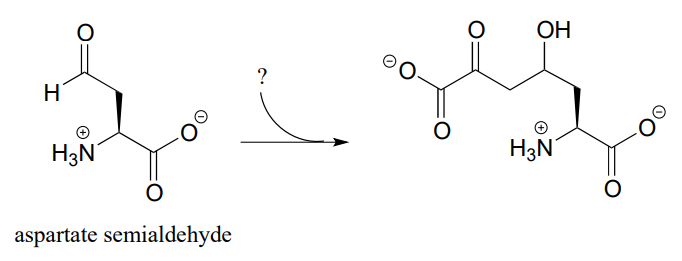
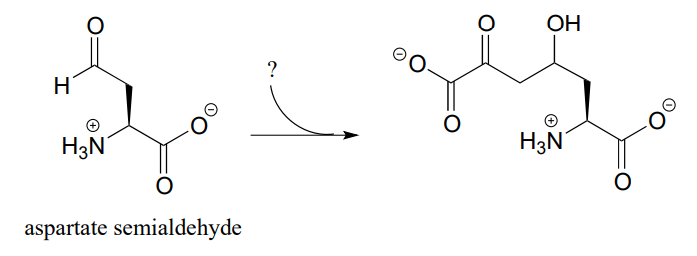
P12.5: The following step in the biosynthesis of lysine makes a connection between aspartate semialdehyde and a common metabolic intermediate. Identify the intermediate, and propose a mechanism for the reaction.
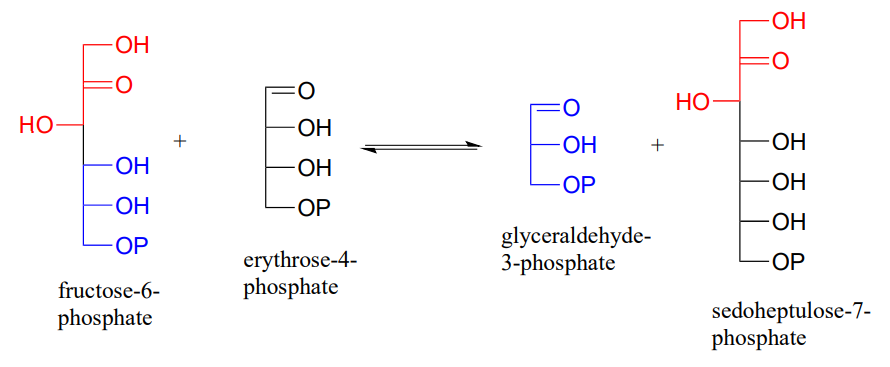
P12.6: Sugar-interconverting transaldol reactions play an important role in sugar metabolism. In a transaldolase reaction, a ketose (e.g. fructose-6-phosphate) first breaks apart in a retro-aldol step to release an aldose (e.g. glyceraldehyde-3-phosphate) from the active site. Then, in a forward aldol step, a second aldose (e.g. erythrose-4-phosphate) enters the active site and connects to what remains from the original ketose (the red part in the figure below) to form a new ketose (e.g. sedoheptulose-7-phosphate). Transaldolase enzymes generally have a lysine in the active site that is covalently bound to the substrate throughout the reaction cycle.
Draw curved-arrow diagrams showing
- the carbon-carbon bond breaking step of the reaction cycle
- he carbon-carbon bond forming step.
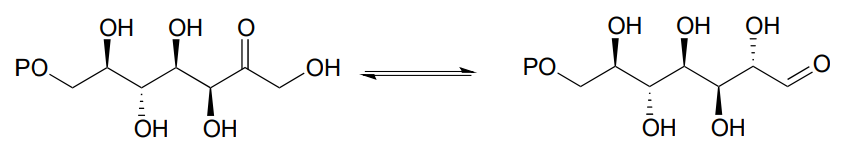
P12.7: Scientists are investigating the enzymatic reaction below, which is part of the biosynthesis of the outer membrane of gram-negative bacteria, as a potential target for new antibiotic drugs. Draw a likely mechanism for the reaction. (J. Biol. Chem. 2008, 283, 2835).
P12.8: The reaction below, catalyzed by the enzyme malate synthase, is part of the glyoxylate cycle of plants and some bacteria. It is the glyoxylate cycle that allows these organisms to convert acetyl CoA, derived from the metabolism of oils, into glucose.
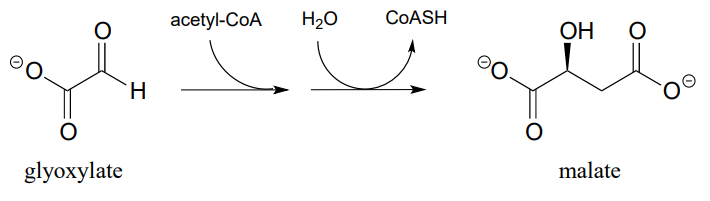
- Propose a mechanism.
- Predict the signals you would expect to see in a \(^1H-NMR\) spectrum of malate.
P12.9: The reaction below, from the biosynthetic pathway for the amino acid tryptophan, is dependent upon a coenzyme that we learned about in an earlier chapter. Based on the reaction, identify this coenzyme and propose a mechanism.
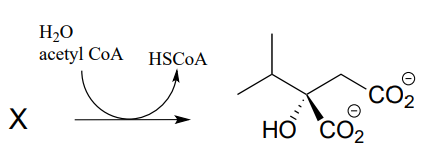
P12.10: In the biosynthesis of leucine, acetyl \(CoA\) condenses with another metabolic intermediate ‘X” to form 1-isopropylmalate (EC 2.3.3.13). Give the structure for substrate X, and provide a mechanism for the reaction.
P12.11:
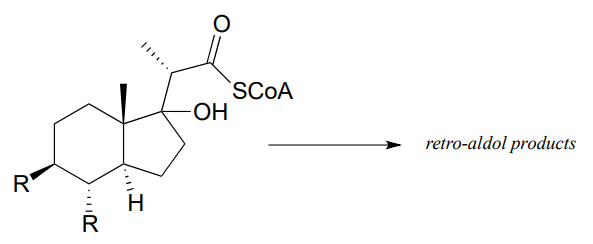
- Mycobacterium tuberculosis, the microbe that causes tuberculosis, derives energy from the metabolism of cholesterol from infected patients. The compound below is predicted to be an intermediate in that metabolic pathway, and to undergo a retro-aldol cleavage reaction. Predict the retro-aldol products and show the mechanism involved. (Crit. Rev. Biochem. Mol. Biol. 2014, 49, 269, fig 5).
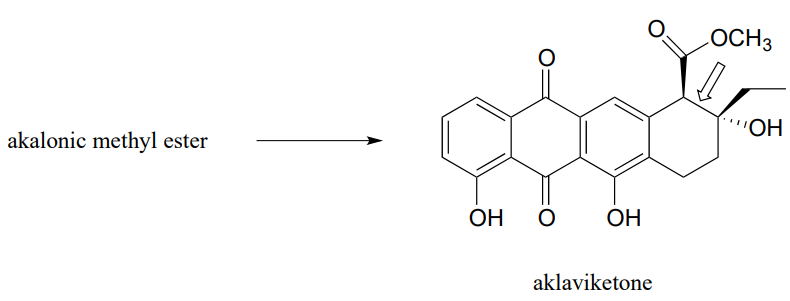
- Polyketides are a structurally diverse class of biomolecules produced by almost all living things. Many drugs are derived from polyketide precursors. The cancer drug doxorubicin (trade name Adriamycin) is derived from a bacterial polyketide called rhodomycinone. Aklaviketone, an intermediate in the biosynthesis of rhodomycinone, is derived in a single enzymatic step from akalonic methyl ester, in a reaction in which the carbon-carbon bond indicated by an arrow is formed. Predict the structure of akalonic methyl ester.
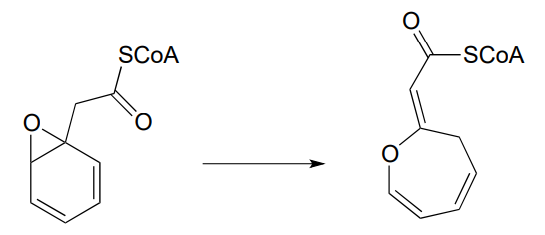
P12.12: The unusual isomerization reaction shown below has been reported recently to occur in some bacteria. Propose a mechanism that begins with formation of an enolate intermediate. (J. Biol. Chem. 2012, 287, 37986).
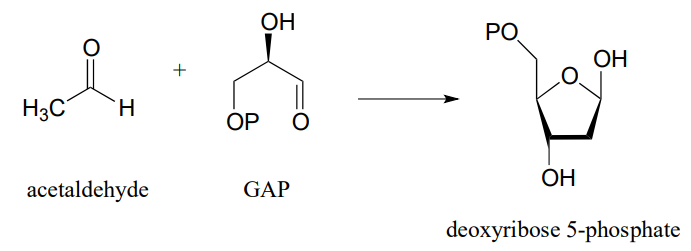
P12.13: The reactions in parts a) and b) below both proceed through lysine-linked enamine intermediates. Show the carbon-carbon bond forming step for each reaction. Hint: you will want to consider the straight-chain (ie. aldose/ketose) form of the sugars in both cases.
- (J. Bacteriol. 2004, 186, 4185)
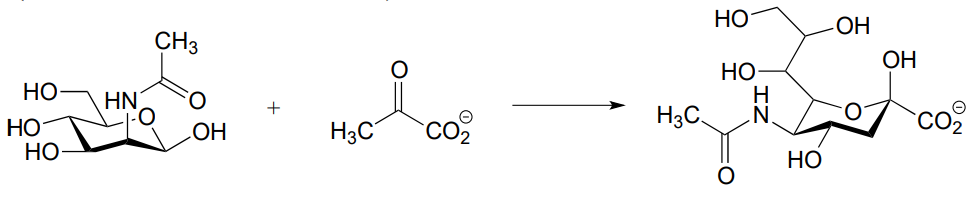
- (J. Biol. Chem. 2011, 286, 14057)
P12.14: Suggest a mechanism for the following transformation from aromatic amino acid biosynthesis (EC 4.2.3.4). Hint – only two mechanistic steps are required.