11.6: Acyl Phosphates
- Page ID
- 106362
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Thioester formation
Thioesters, which are themselves quite reactive in acyl substitution reactions (but less so than acyl phosphates), play a crucial role in the metabolism of fatty acids The ‘acyl X group’ in a thioester is a thiol.
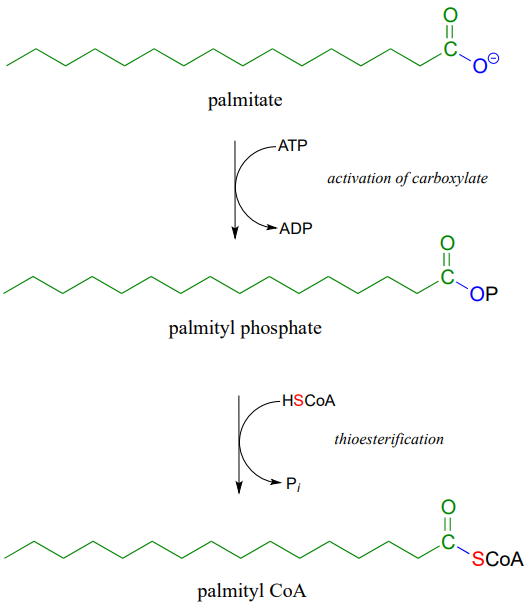
Coenzyme A is a thiol-containing coenzyme that plays a key role in metabolism. Coenzyme A is often abbreviated 'HSCoA' in order to emphasize the importance of the thiol functionality.
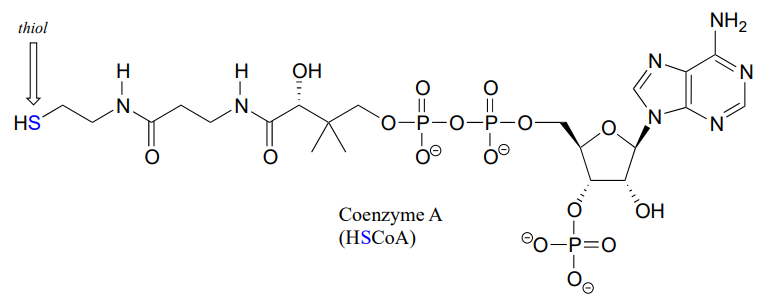
Coenzyme A serves as a 'carrier' group in lipid biosynthesis, and is attached by a thioester linkage to growing fatty acid chains. Palmityl is shown below as an example of a typical fatty acyl-CoA thioester.
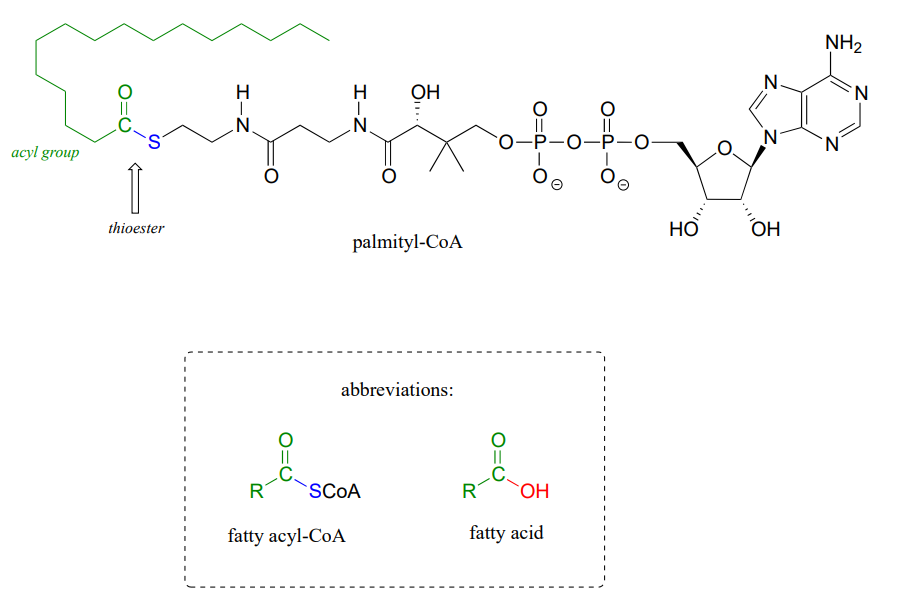
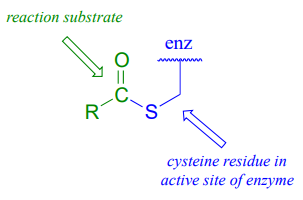
As we look at reactions involving thioesters in this and future sections, we will frequently see Coenzyme A playing a key role. We will also see the formation and breaking of thioester linkages between an acyl group and other thiol-containing species, such as a cysteine residue on the enzyme:
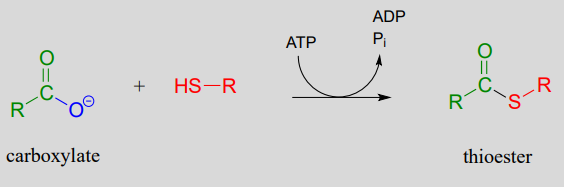
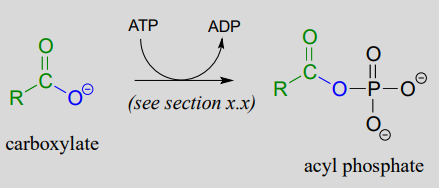
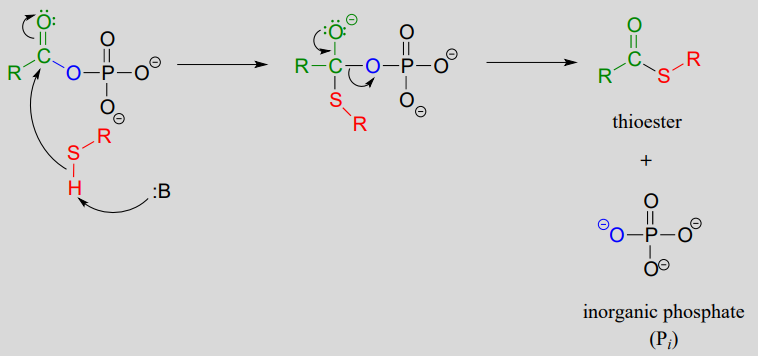
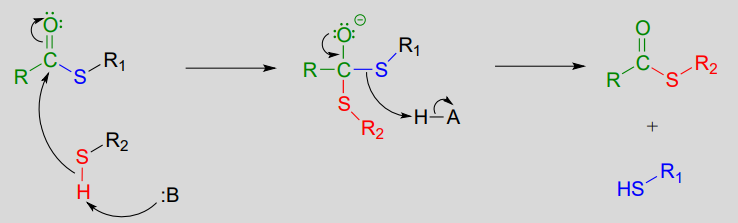
The term 'thioesterification' refers to the formation of a thioester functional group. In a typical biochemical thioesterification reaction, a carboxylate is first converted into an acyl phosphate (in other words, it is activated), then the acyl phosphate undergoes an acyl substitution reaction with a thiol nucleophile.
Thioesterification reaction:
Mechanism:
1. activation phase:
2. acyl substitution phase:
Fatty acids such as palmitate , from fats and oils in your food, are converted to a coenzyme A thioester prior to being broken down by the fatty acid degradation pathway.
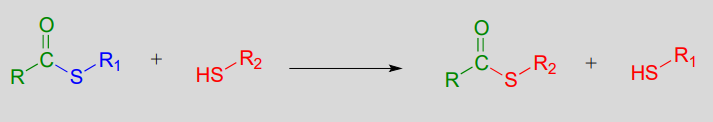
A transthioesterification reaction is a thioester to thioester conversion - in other words, an acyl group is transferred from one thiol to another.
Transthioesterification:
Mechanism:
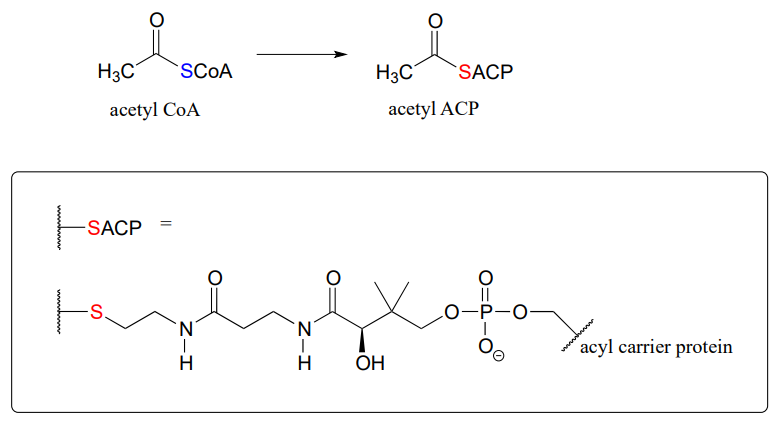
For example, when your body synthesizes fatty acids, the two-carbon fatty acid 'building block' acetyl CoA is first converted to acetyl ACP (EC 2.3.1.38). ACP is an abbreviation for 'Acyl Carrier Protein', a modified protein with a thiol-containing prosthetic group attached to one of its serine side chains. Throughout the fatty acid chain elongation process, the growing hydrocarbon chain remains linked to ACP.
The pyruvate dehydrogenase complex (EC 1.2.4.1) catalyzes one of the most central of all central metabolism reactions, the conversion of pyruvate to acetyl-CoA, which links the gycolytic pathway to the citric acid (Krebs) cycle. The reaction is quite complex, and we are not yet equipped to follow it through from start to finish (we will finally be ready to do this in section 17.3). The final step, however, we can understand: it is a transthioesterification, involving a dithiol coenzyme called dihydrolipoamide and coenzyme A. Given the information below, draw out a reasonable mechanism for the reaction.

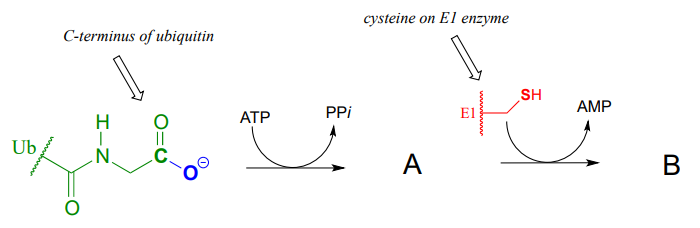
Ubiquitin is a protein which plays a key role in many cellular processes by reversibly attaching to other proteins, thus altering or regulating their function. Recently, a team of researchers uncovered details of the mechanism by which ubiquitin (abbreviated Ub) is transferred by the ubiquitin activating enzyme (abbreviated E1) to target proteins. In the first part of this process, the carboxy terminus of ubiquitin is linked to a cysteine side chain on E1, as shown in the incomplete reaction sequence below. Complete the figure by drawing the structures of species A and B.
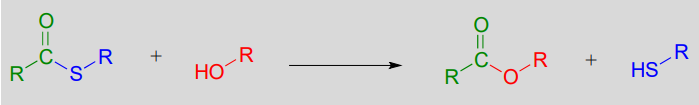
Formation of esters
Esterification refers to the formation of a new ester functional group.
In a typical biochemical esterification, a thioester is subjected to nucleophilic attack from an alcohol, leading to the formation of an ester and a thiol.
Esterification reaction (from thioester):
Mechanism:
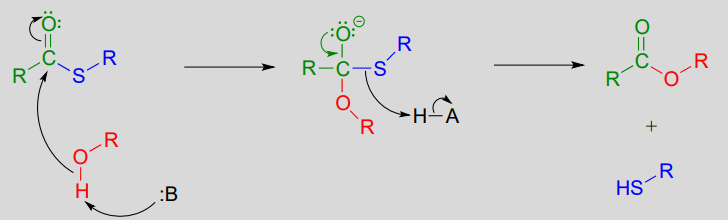
The reaction below is from the synthesis of triacylglycerol, the form in which fat is stored in our bodies.
Phase 1 (transthioesterification):

Phase 2 (esterification):

The reaction, catalyzed by monoacylglycerol acyltransferase (EC 2.3.1.22), begins (phase 1 above) with a preliminary transthioesterification step in which the fatty acyl group is transferred from coenzyme A to a cysteine residue in the active site of the enzyme. Recall that it is a common strategy for enzymes to first form a covalent link to one substrate before catalyzing the 'main' chemical reaction.
In phase 2 of the reaction, the fatty acyl group is now ready to be transferred to glycerol, trading its thioester linkage to the cysteine for a new ester linkage to one of the alcohol groups on glycerol.
An esterification reaction has tremendous importance in the history of drug development, a story that we heard in the introduction to this chapter. The discovery of penicillin was arguably one of the most important events in the history of modern medicine. The key functional group in penicillin is the four-membered lactam (recall that a lactam is a cyclic amide).
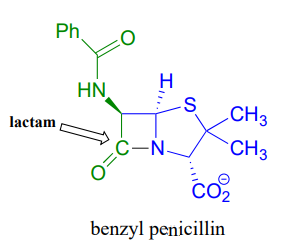
Penicillin, and later generations of antibiotic drugs, have saved countless lives from once-deadly bacterial infections. The elucidation of the chemical mechanism of penicillin action was also a milestone in our developing understanding of how drugs function on a molecular level. We now know that penicillin, and closely related drugs such as ampicillin and amoxycillin, work by inhibiting an enzyme that is involved in the construction of the peptide component bacterial cell walls. The details of the wall-building reaction itself are outside the scope of this discussion, but it is enough to know that the process involves the participation of a nucleophilic serine residue in the active site of the enzyme. The penicillin molecule is able to enter the active site, and once inside, the lactam group serves as an electrophilic 'bait' for the nucleophilic serine:
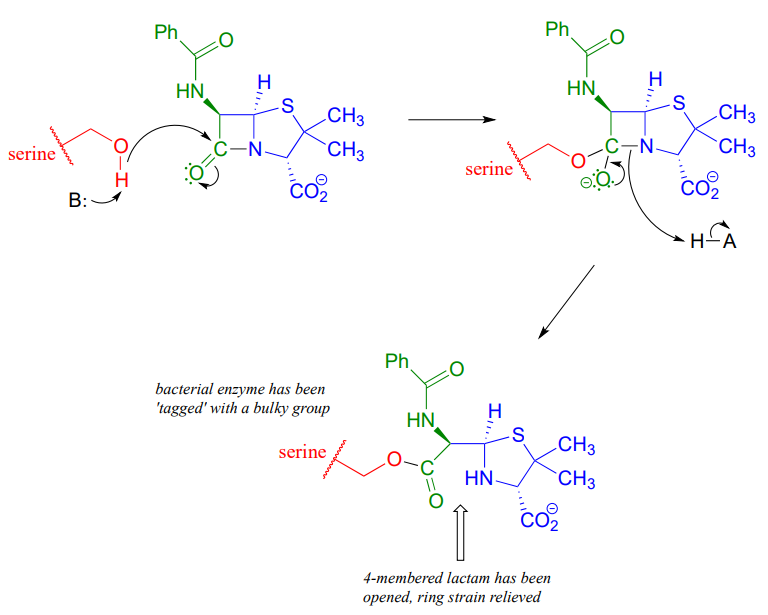
Although you might expect that an amide-to-ester conversion such as what is shown above would be energetically unfavorable based on the reactivity trends we have learned, this lactam is in fact much more reactive than an ordinary amide group due to the effect of ring strain: recall from section 3.2 that four-membered rings are highly strained, and considerable energy is released when they are opened.
Ring strain also accounts for why penicillin has a tendency to degrade: when in contact with water, the lactam will spontaneously hydrolyze over time, which opens the ring and forms a carboxylate group.
Unfortunately, many strains of bacteria have acquired an enzyme called \(\beta \)-lactamase (EC 3.5.2.6), that catalyzes rapid hydrolysis of the lactam ring in penicillin-based drugs, rendering them inactive. These bacteria are consequently resistant to penicillin and related antibiotics. As you are probably aware, the evolution of drug resistance in bacteria is a major, world-wide health problem, and scientists are engaged in a constant battle to develop new antibiotics as the older ones become less and less effective.
In a transesterification reaction, one ester is converted into another by an acyl substitution reaction.
Mechanism for a transesterification reaction:
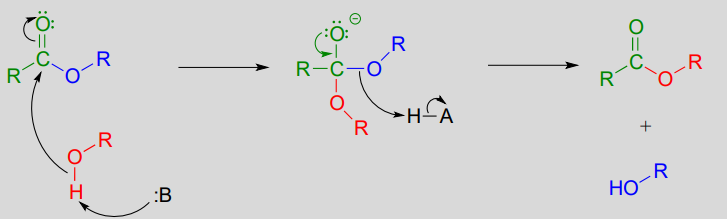
If studying organic chemistry sometimes gives you a headache, you might want to turn to a transesterification reaction for help. Prostaglandins are a family of molecules that promote a wide range of biological processes, including inflammation. Acetylsalicylic acid, commonly known as aspirin, acts by transferring - through a transesterification reaction - an acetyl group to a serine residue on the enzyme responsible for the biosynthesis of prostaglandin H2 (one member of the prostaglandin family).
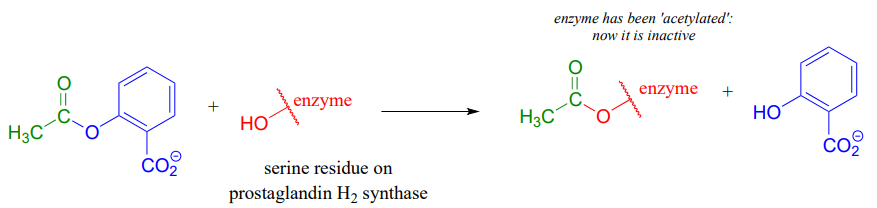
Acetylation of this serine blocks a channel leading to the active site, effectively shutting down the enzyme, impeding prostaglandin production, and inhibiting the inflammation process that causes headaches.
In section 11.8, we will see two laboratory acyl substitution reactions that lead to the formation of aspirin and ibuprofen.
Discuss the key structural feature of aspirin that makes it so effective at transferring its acetyl group - in other words, why is the ester group in aspirin more reactive than a typical ester?
Amide formation
An activated carboxylate group (in other words, acyl phosphate or acyl-AMP) can be converted to an amide through nucleophilic attack by an amine.
Mechanism for amide formation:
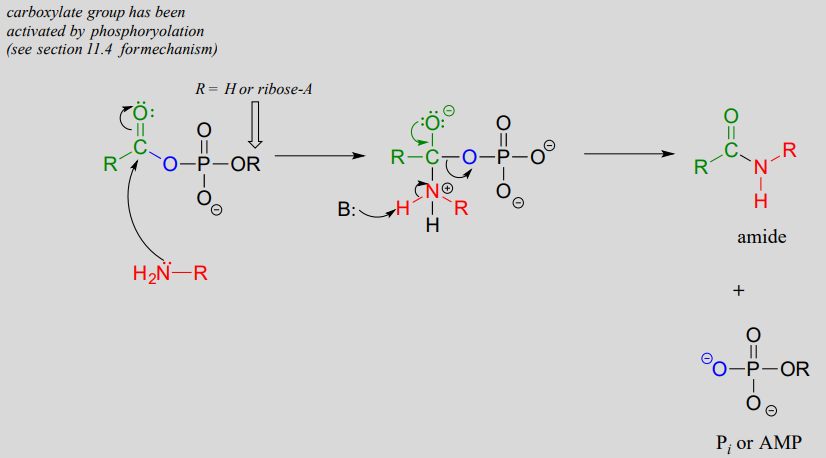
The amino acid biosynthesis pathways provide examples of amide formation in biology. The amino acid glutamine is synthesized in most species by converting the carboxylate side chain of glutamate (another amino acid) to an amide, after first activating the carboxylate by monophosphorylation: (EC 6.3.1.2)

A similar process takes place in the synthesis of asparagine from aspartate, except that the activated carboxylate in this case is an acyl-AMP:
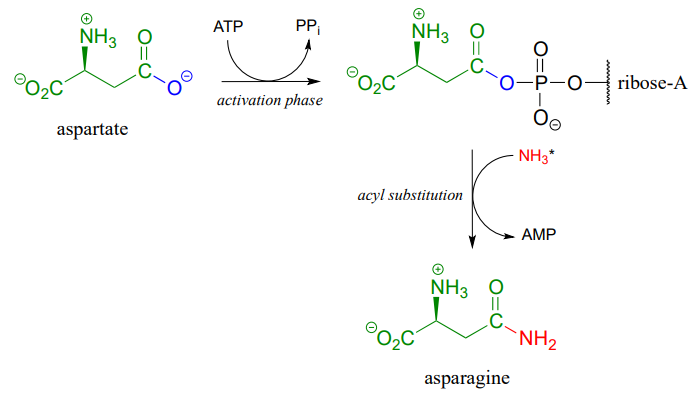
*In the asparagine synthesis reaction, the ammonia nucleophile actually comes from hydrolysis of a glutamine molecule.
A enzyme in bacteria is thought to be responsible for resistance to a class of antibiotics that includes apramycin, ribostamycin and paromomycin. The enzyme catalyzes acetylation of the antibiotic compound with acetyl-CoA as an additional substrate. The structure of acetylated apramycin is shown below.
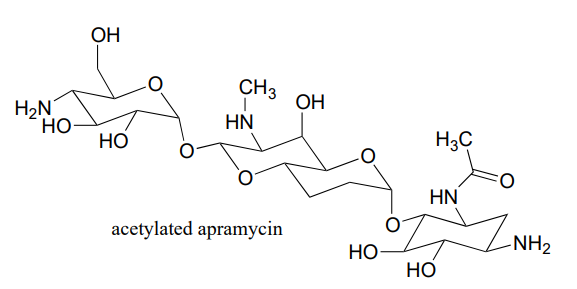
- a) Identify the acetyl group that has been transferred to apramycin, (and thus inactivating it).
- b) What functional group acts as an acetyl group donor? What functional group acts as an acetyl group?
- c) What is the coproduct of the reaction?


