9.E: Phosphate Transfer Reactions (Exercise)
- Page ID
- 106568
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)In all of the problems that follow, feel free to use appropriate abbreviations when drawing structures. However, always be sure not to abbreviate regions of a structure which are directly involved in bond-breaking or bond-forming events.
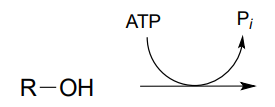
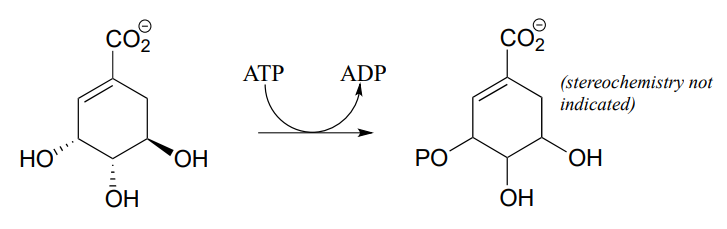
P9.1: Draw a likely mechanism for reaction catalyzed by shikimate kinase (EC 2.7.1.71) in the aromatic amino acid biosynthesis pathway). Stereochemistry of the product is not indicated in the figure below - in your mechanism, show the stereochemistry of the product, and explain how you are able to predict it from your knowledge of kinase reactions.
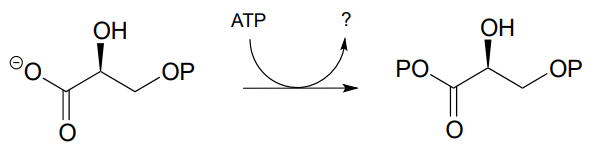
P9.2: Draw a likely mechanism for the following reaction (EC 2.7.2.3) in the gluconeogenesis pathway, and predict what compound is indicated by the question mark.
P9.3:
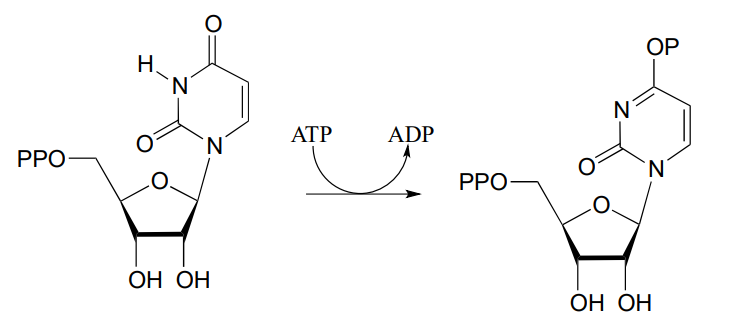
- Draw a likely mechanism for the following reaction (EC 6.3.4.2) from ribonucleotide biosynthesis. Hint: what is the nucleophilic group? How could the enzyme increase it's nucleophilicity?
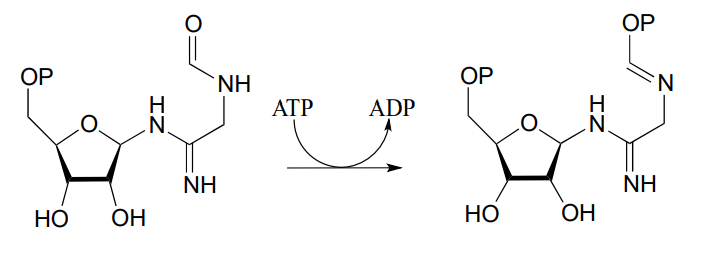
- Draw a mechanism for the following reaction, also from ribonucleotide biosynthesis (EC 6.3.3.1):
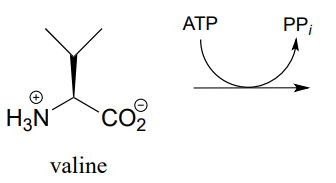
P9.4:
- The carboxylate group on the amino acid valine is activated in an early step in the biosynthesis of the antibiotic penicillin. Predict the product of this reaction, and draw the likely intermediate of the phosphate group transfer reaction.
P9.5: The reaction below is an early step in the synthesis of tyvelose, a sugar found on the surface of some pathogenic bacteria. Notice that CTP plays the role of the phosphate group donor in this case, rather than ATP.
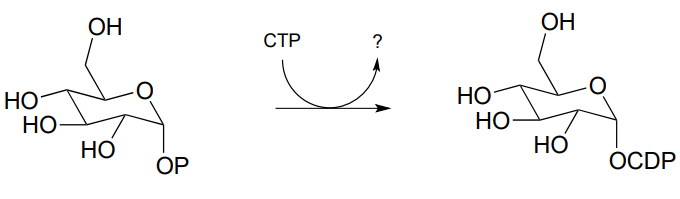
Draw a mechanism for the reaction, and indicate the second product that is released by the enzyme. (J. Biol. Chem. 2005, 280, 10774)
P9.6: Draw the likely product of the following hypothetical phosphate group transfer reactions. Specify which phosphate group of ATP is the electrophile in each case.
P9.7: The figure below illustrates an experiment in which a reaction catalyzed by an E. coli enzyme was run in isotopically labeled water.

- The researchers concluded that the reaction was not a phosphate group transfer. Explain their reasoning.
- Draw the products that would be expected if the reaction actually did proceed by a phosphate transfer mechanism (be sure to show stereochemistry and the location of the \(^{18}O\) atom).
P9.8: The reaction below proceeds with a direct attack by a water molecule on the substrate, but the hydrolysis could be expected to proceed through two possible mechanisms. Draw two possible mechanisms for the reaction run in \(H_2^{18}O\). Trace the progress of the \(^{18}O\) 'label' throughout each mechanism to see where it ends up: this should indicate to you how the two mechanisms could be (and in fact were!) distinguished experimentally.
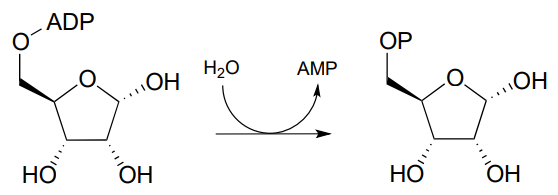
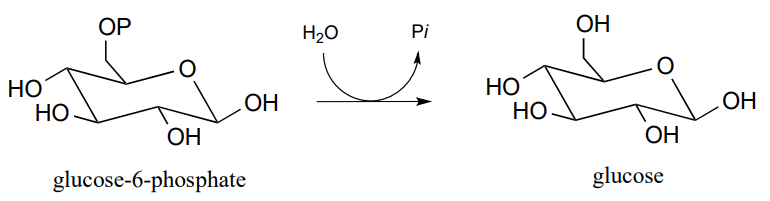
P9.9: Glucose-6-phosphate is dephosphorylated to glucose in the last step of the gluconeogenesis pathway (EC 5.3.1.9). The reaction is not a direct hydrolysis: like the phosphotyrosine phosphatase reaction we saw in this chapter it involves formation of a phosphoenzyme intermediate, but in this case the enzyme residue acting as the initial phosphate acceptor is an active site histidine rather than an asparate. Given this information, propose a likely mechanism for the reaction.
P9.10: (This question assumes a basic knowledge of DNA structure and the idea of supercoiling). DNA topoisomerase enzymes catalyze the temporary 'nicking' of one strand of double-stranded DNA, which allows supercoiled DNA to 'unwind' before the nicked strand is re-ligated. During the unwinding process, the 5' end of the nicked strand is transferred to a tyrosine in the enzyme's active site, effectively holding it in place while the 3' end rotates. Overall, the stereochemical configuration of the bridging phosphate is retained. Propose a likely mechanism for this nicking and re-ligating process. (Biochemistry 2005, 44, 11476.)
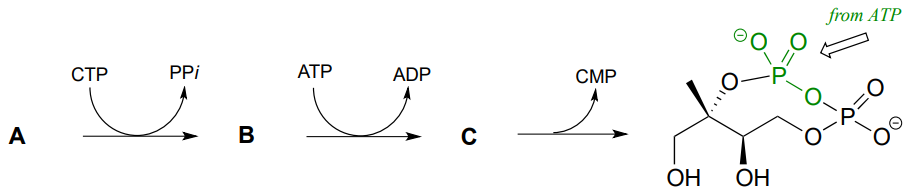
P9.11: Pictured below is a series of phosphate group transfer steps in the early part of isoprenoid biosynthesis in bacteria. With the knowledge that the atoms in green are derived from ATP, predict the structures of compounds A, B and C. (EC 2.7.7.60, EC 2.7.1.148, EC 4.6.1.12)
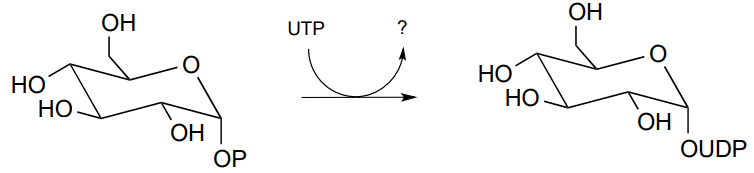
P9.12: The reaction below shows the synthesis of glucose-UDP, an important intermediate in carbohydrate biosynthesis. Notice that UTP (instead of ATP) is the phosphate donor. Identify the by-product denoted below by a question mark.
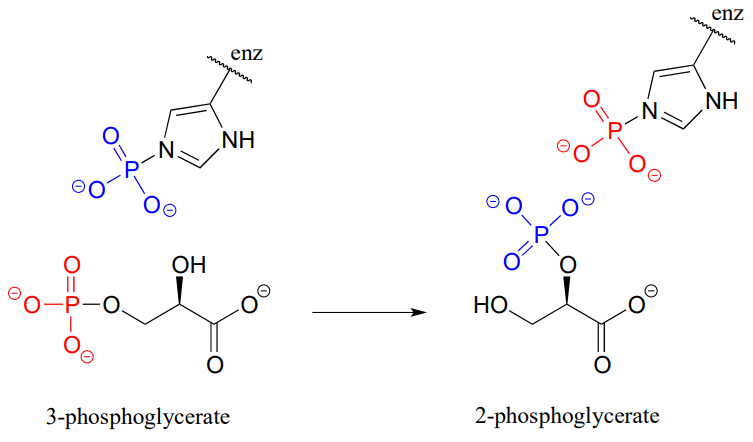
P9.13: Isomerization of 3-phosphoglycerate to 2-phosphoglycerate (EC 5.4.2.1, a reaction in glycolysis) has been shown to occur with the participation of a phosphohistidine residue in the enzyme's active site. The two phosphate groups are distinguished in the figure below by color. With this information, propose a mechanism for the reaction.
P9.14: The gluconeogenesis (sugar-building) pathway enzyme glucose-6-phosphatase catalyzes an indirect phosphate hydrolysis reaction with a phosphohistidine intermediate ('indirect hydrolysis' in this context means that a water molecule does not directly attack glucose-6-phosphate).
Researchers wanted to confirm that the hydrolysis in this reaction is indirect, rather than direct. It turns out that the same enzyme is also capable of catalyzing the transfer of the phosphate group from glucose-6-phosphate to the hydroxyl group on carbon #6 of another glucose molecule (instead of to water, which is the natural reaction). The enzyme-catalyzed transfer of phosphate between two glucose substrates is reversible.
The researchers incubated the enzyme with labeled glucose-6-phosphate, in which in the phosphate center was chiral (with the R configuration) due to the incorporation of \(^{17}O\) and \(^{18}O\) isotopes. They also included a high concentration of glucose in the reaction mixture, which ensured that the glucose-to-glucose transfer reaction predominated and hydrolysis (the 'natural' reaction) did not take place. After allowing the reaction to reach equilibrium, they isolated the glucose-6-phosphate and looked at the configuration of the phosphate group.
Given what you have just learned about the enzyme mechanism, predict what the researchers found in this experiment, explain your prediction, and draw the appropriate structure(s), including stereochemistry. Assume that the glucose-to-glucose mechanism is identical to the hydrolysis mechanism, aside from the identity of the ultimate phosphate acceptor.