1.29: Metabolic Organic Reactions
- Page ID
- 81795
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Beta-Oxidation of Fatty Acids
Today we're going to examine a selection of processes which occur in metabolism. We will focus on comparing these reactions to reactions we have already studied. In particular we will see that the reactions which break carbon-carbon bonds are just reverse versions of the aldol and Claisen condensations which we have studied earlier. Keep in mind that while we are looking for connections between these reactions and familiar organic reactions, all steps in these schemes are catalyzed by enzymes.
The two processes we are going to study are both catabolic processes, that is, they are processes that break down and oxidize larger molecules to produce smaller molecules and energy. The first, beta-oxidation, is a key part of the process by which fatty acids are broken down to acetate. [Acetate is the conjugate base of acetic acid. Since a neutral pH is more basic than the pKa of acetic acid (~5), in neutral solution acetic acid is predominantly ionized and acetate is the major form present.] The overall scheme of beta-oxidation looks like this:
From this we can see that the outcome of a beta-oxidation "event" is that two carbon atoms are cleaved from a fatty acid. The bond broken is between the alpha and beta carbons. (This accounts for the term "beta-oxidation.") The gamma carbon shows up in the product as a carboxylic acid. This carboxylic acid, two carbons shorter than its "parent," can be shortened by another trip through the beta-oxidation process, with the production of another molecule of acetate and a new fatty acid, again two carbons shorter. That the reactions all occur at the carboxylic acid end of the molecule rather than at the CH3 end is not surprising, since a fundamental idea of organic chemistry is that reactions occur at functional groups rather than elsewhere.
Prior to the commencement of the actual beta-oxidation cycle, the carboxylic acid end of the fatty acid is esterified with the SH group of coenzyme-A. This reaction is discussed in more detail in Sec 20.3A in Brown. Here is the scheme as it more realistically looks with the coenzyme-A included.
Now let's look at the individual reactions of this process. (Notice the slightly different notation scheme. The principal reactant and product are connected by a straight arrow. The necessary reagents and their byproducts are connected by a curved arrow.) The first reaction results in the removal of hydrogen atoms from the alpha and beta carbo atoms. Its effects are opposite to those of hydrogenation of a double bond. The removal of hydrogen atoms makes this an oxidation reaction. The oxidizing agent is a molecule called "FAD" (for flavine adenine dinucleotide). We'll look at its structure later.
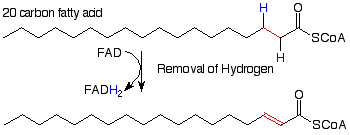
Water is then added to the alkene pi bond which results from the first reaction. This is analogous to the addition of water to alkenes we studied recently. Since we know that a carbon alpha to a carbonyl group is a rather nuclophilic place (remember enolates?), it makes sense that the electrophilic hydrogen from water would add there and the nucleophilic OH would add at the beta carbon.
As soon as we see the third step,
we recognize the oxidation of a a secondary alcohol to a ketone. This is a reaction for which we used chromium-6 reagents earlier, but now the oxidizing agent is NAD+ (nicotine adenine dinucleotide cation). The hydrogen attached to the OH-bearing carbon is transferred to the NAD+, and the OH hydrogen comes away as an H+.
The final step is the actual cleavage of the bond between the beta-carbon and the gamma carbonyl group. To put this in context, we have to think about it in reverse. When we do that, we see a pattern which is identical to the Claisen condensation.
The conclusion is that the final step in the beta-oxidation cycle is a reverse Claisen condensation. Like the Claisen condensation itself, this step is possible because the enolate ion obtained as the acetate fragment breaks away is stabilized by resonance. This enolate ion is neutralized by a proton source (acid). The shortened fatty acid is released from the enzyme as CoA?SH replaces the sulfur of the enzyme. This last step goes through a tetrahedral intermediate (not shown) as we would expect for a reaction which converts one carboxylic acid derivative to another.
The acetyl-coenzyme-A formed in this cycle enters the tricarboxylic acid cycle where it is oxidized to two molecules of CO2. The NADH and FADH2 produced in beta-oxidation and the tricarboxylic acid cycle enter a process called oxidative phosphorylation which results in the formation of ATP (adenosine triphosphate) for use in providing energy within the cell. We will not take up these latter processes, but they are an important part of a biochemistry course.
Glycolysis
Our next topic is glycolysis. This is the conversion of glucose to pyruvate. In the larger scheme of things the pyruvate produced is then converted to acetate, which like the acetate from beta-oxidation of fatty acids, enters the tricarboxylic acid cycle. Again, we will be looking at the reactions involved trying to find analogies in organic reactions we have studied. The chart in Fig 20.2 (p 571) in Brown lays out the whole scheme of glycolysis. Please refer to that chart to identify the reactions we are considering.
Reaction 1 is an esterification. It resembles the conversion of an alcohol to an ester by an acid chloride. In Reaction 1, ATP is used rather than an acid chloride, and the result is an ester of phosphoric acid rather than an ester of a carboxylic acid.
Reaction 2 is formally an internal oxidation-reduction reaction. The carbonyl group of glucose is reduced to a primary alcohol while the OH group at carbon-2 of glucose is oxidized to a ketone. The reaction goes through an enol as shown on p 572 of Brown.
Reaction 3 is similar to reaction 1, the formation of a phosphate ester (this is called a phosphorylation reaction).
Reaction 4 involves cleavage of a carbon-carbon bond and is the reaction in which the carbon skeleton changes from a six carbon chain to two three carbon chains. The first thing to notice is that the carbon-carbon bond broken joins a carbon which is alpha to a carbonyl group to one which is beta. This suggests that enol and enolate reactivity is going to be important. Like we did with the beta-oxidation of fatty acids, we can see a familiar reaction if we think about this one in the reverse direction, that is, look at it as a bond-making reaction rather than a bond-breaking reaction. If we ignore the groups which don't change, we can see that our reaction is a reverse aldol addition.
In more detail, the reverse aldol steps in this reaction are preceded by conversion of the carbonyl group of fructose 1,6-bisphosphate to an imine, and followed by the hydrolysis of that imine back to a carbonyl group and the amino group. The amino group is attached to the enzyme which catalyzes this reaction, and the formation of the imine helps anchor the fructose 1,6-bisphosphate molecule to the enzyme in the proper position to bring the bases and acids on the enzyme to the right location for the mechanism to go forward. The sequence of mechanistic steps is:
Reaction 5 converts dihydroxy acetone phosphate to glyceraldehyde 3-phosphate. This is like the reverse of reaction 2 in that it goes through an enol intermediate to oxidize an OH group to a carbonyl on one atom and reduce a carbonyl to an OH on the adjacent carbon. The sequence is on p 574 in Brown. At this point in glycolysis a glucose molecule has been converted to two molecules of glyceraldehyde 3-phosphate.
Reaction 6 is an oxidation of the aldehyde in glyceraldehyde 3-phosphate to a carboxylic acid. The odxidizing agent is NAD+, which is reduced to NADH. The process also includes formation of an anhydride between the newly formed carboxylic acid and a molecule of monohydrogen phosphate ion. This anhydride is quite reactive (since phosphate is a stable anion, it's a good leaving group much like chloride) along the lines of an acid chloride.
Reaction 7 exploits the reactivity of this mixed anhydride by using it to transfer the phosphate to ADP. The resulting ATP is used as an energy source for many cell processes, so this step in glycolysis directly produces energy for the cell.
Reaction 8 shifts the phosphate group from the OH on carbon 3 to the OH on carbon 2. It is like an ester hydrolysis at one carbon and an esterification at the other.
Reaction 9 is a dehydration reaction. It resembles the dehydration of cyclohexanol to cyclohexene we did in lab, although the conditions in a cell are much milder since enzyme efficiencies make it unnecessary to employ strong acids and heat. The product is an enol phosphate (the phosphoric acid of a ketone).
Reaction 10 does two things. Like reaction 7 transfers a phosphate to ADP, making ATP, and it produces the enol form of pyruvate which rapidly equilibrates to the keto form.
If we add up all the balanced reactions, we find that one glucose molecule, two ADP molecules, two NAD+ molecules, and two hydrogen phosphate molecules have been converted to two pyruvates, two ATP's, two NADH's and two hydronium ions.
Fates of Pyruvate
What happens to the pyruvate? This depends on the local conditions. In muscle tissue there may be a limited supply of oxygen (due to the demands of exercise outpacing the body's capacity to supply oxygen to the tissue). This means that the NADH produced in glycolysis not oxidized back to NAD+. Glycolysis requires NAD+, so it would stop and along with it production of energy in the form of ATP. This can be circumvented by lactate fermentation, which reduces pyrvate to lactate and oxidizes NADH to NAD+. Energy production can continue until the build-up of lactate and acid as a result of this reaction (Brown, p 577) exhausts the muscle.
In yeasts in the absence of oxygen, fermentation to alcohol occurs. This is the basis of the fermentation processes which produce beverage alcohol in beer and wine. The byproduct is CO2 which is responsible for the carbonation of beer and sparkling wines. If the yeast is used in baking, the CO2 expands the dough to produce the "rise" of bread dough. In this case the alcohol is the byproduct and it is largely driven off by the heat of baking. It is also responsible for much of the pleasant odor of baking bread.
If there is a good oxygen supply, pyruvate is oxidized to acetyl CoA and CO2. As we have seen now several times, the oxidizing agent is NAD+ which is reduced to NADH. This reaction is more complex than it looks at first glance, but we will leave those complexities for a biochemistry course.


