7.1: Reactions in Water
- Page ID
- 168808
Many of the organic solvents are volatile, flammable, sometimes explosive and have damaging effect to human health or on the environment. Thus, effort has been made to use nonconventional solvents which are not only attractive from economical aspects, they can provide advantages of recovery and recyclability of the catalysts. The section covers the use of water, fluorous solvents, supercritical fluids and ionic liquids as nonconventional solvents.
The use of water as a reaction medium for organic synthesis has attracted much interest in recent years. Because water is the most abundant liquid on the planet, cheap, readily available, non-toxic and non-flammable. This section covers some of the recent developments in asymmetric catalysis that have been performed in water as reaction medium.
Mannich Reaction
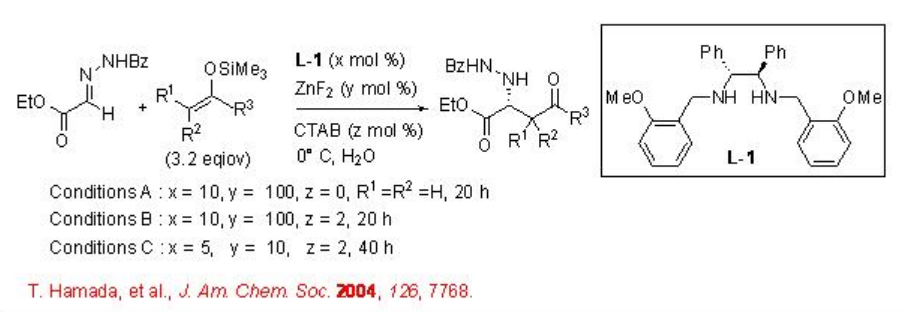
Mannich reaction affords useful route for the synthesis of β -amino ketones and esters that serve as building blocks for the construction of nitrogen containing compounds. The asymmetric version of the reaction has been shown from α -hydrazono ester and silicon enoate using chiral ZnF2 - L-1 complex in aqueous medium (Scheme \(\PageIndex{1}\)). The reaction proceeds without any organic solvents or additives and the presence of cetyltrimethyl ammonium bromide (CTAB) is necessary to accelerate the reaction.
Michel Reaction
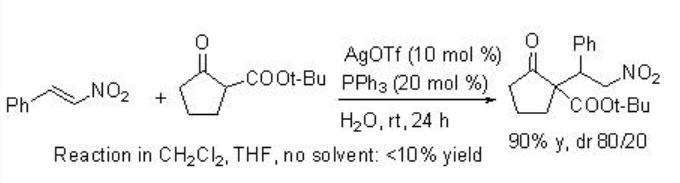
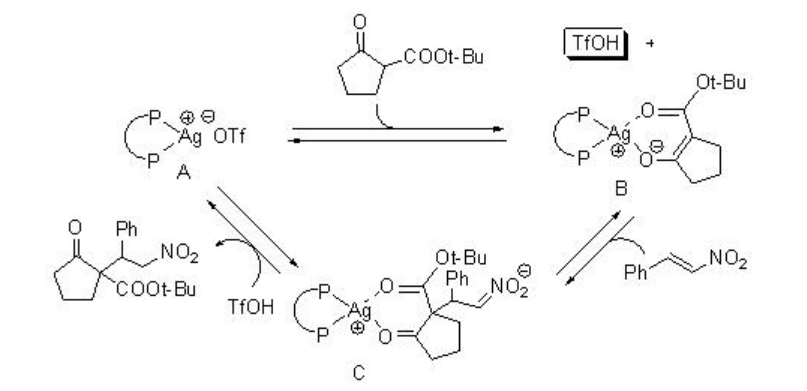
Michel addition of β -ketoesters to nitroalkanes using AgOTf-PPh3 proceeds efficiently in water but not in organic solvents (Scheme \(\PageIndex{2}\)). Regarding the mechanism, the reaction in water becomes heterogeneous, and the metal enolate stays in organic phase, while TfOH is excluded into water phase because of the difference between their hydrophobicity (Scheme \(\PageIndex{3}\)). Thus, the metal enolate B does not have the contact with TfOH, and the reverse reaction from B to A is suppressed. In contrast, in normal organic solvent, the reaction mixture becomes homogeneous and the reverse reaction from B to A is fast.
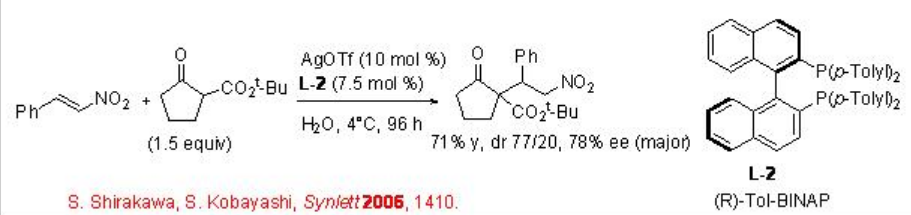
This reaction has been applied for the asymmetric version employing L-2 as chiral source to afford the target products with up to 78% ee (Scheme \(\PageIndex{4}\)).
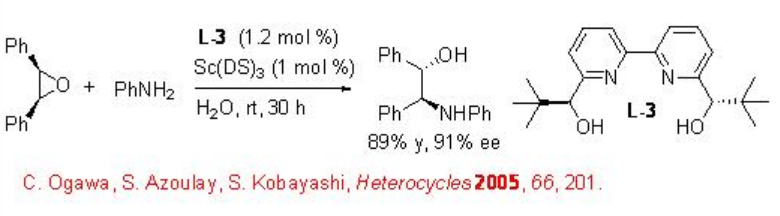
Desymmetrization of Epoxides
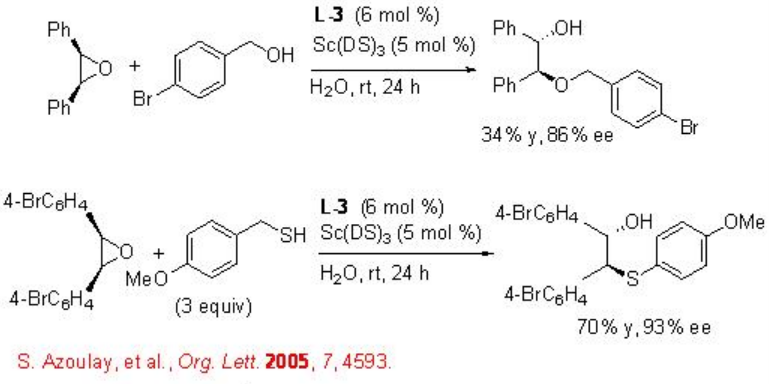
This reaction condition has been subsequently used for the asymmetric desymmetrization of epoxides with nitrogen nucleophiles using L-3 as chiral source (Scheme \(\PageIndex{5}\)). The reaction proceeds with high enantioselectivity and no diol formation has been observed.
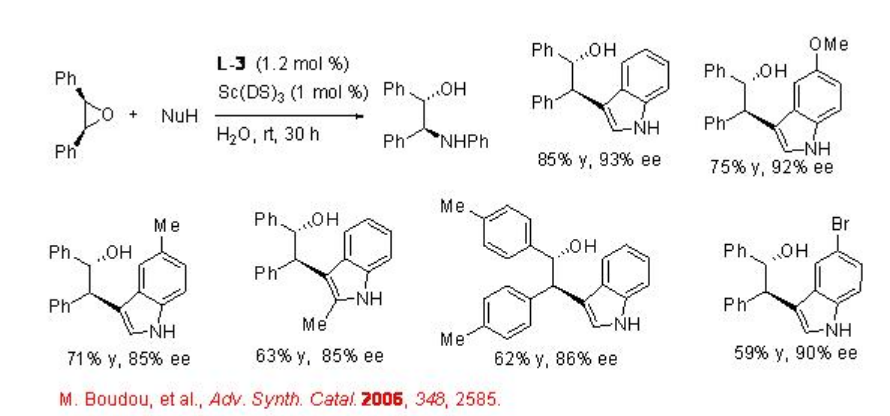
The asymmetric desymmetrization of the epoxides is also successful with indoles, alcohols and thiols with high enantioselectivities (Schemes \(\PageIndex{6}\) and \(\PageIndex{7}\)).
Aldol Reaction
The reaction of silyl enol ethers with aldehydes has been demonstrated using scandium trisdodecylsulfate (Sc(DS)3 ) as a Lewis acid as well as surfactant in water (Scheme \(\PageIndex{8}\)). The reaction is sluggish when Sc(OTf)3 is used as a catalyst. In this process, the formation of stable emulsions takes place.
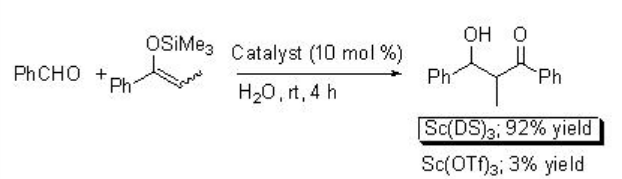
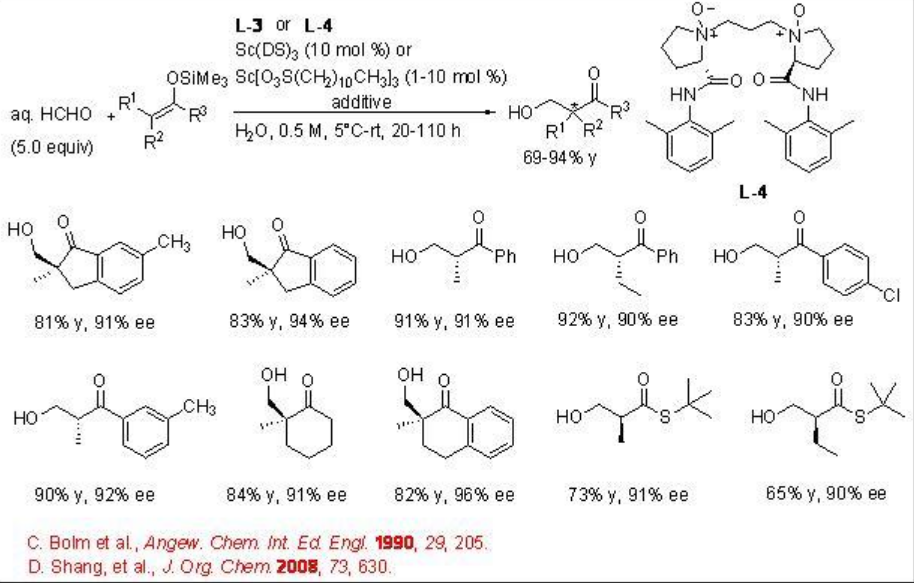
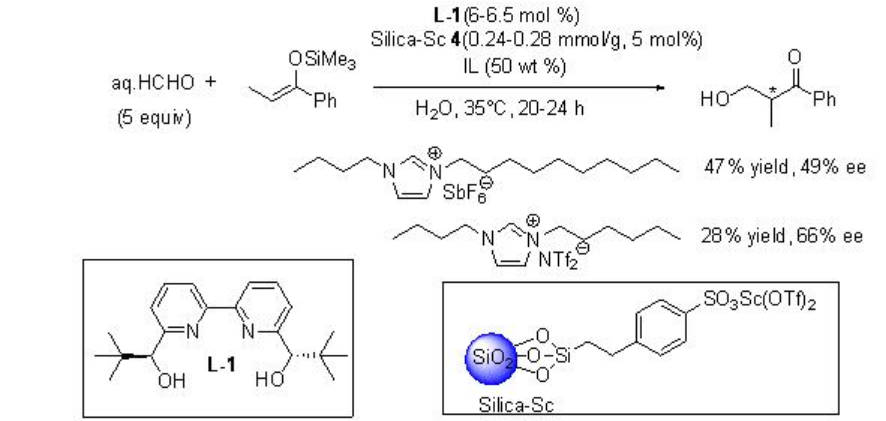
The reaction condition has also been further demonstrated for the hydroxymethylation using aq HCHO with excellent enantioselectivity (Scheme \(\PageIndex{9}\)).
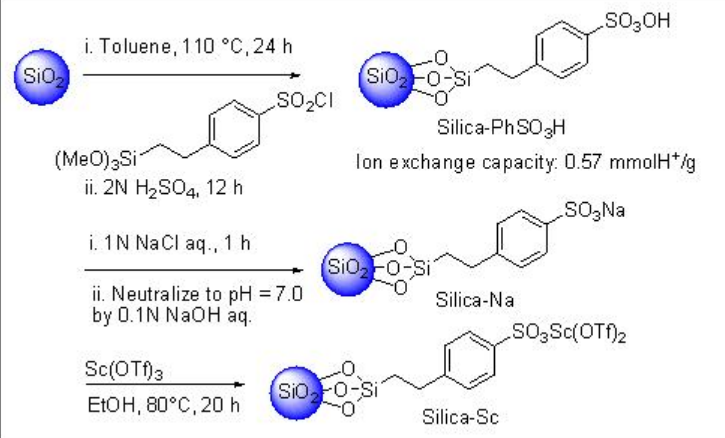
Silica gel-supported scandium (Silica-Sc) with ionic liquid, [DBIm]SBF6, is a heterogeneous catalytic system works efficiently in Mukaiyama aldol reaction in water (Schemes \(\PageIndex{10}\) and \(\PageIndex{11}\)). The reaction proceeds efficiently in water medium compared to that in organic solvents, without solvent or in the absence of ionic liquid (IL).
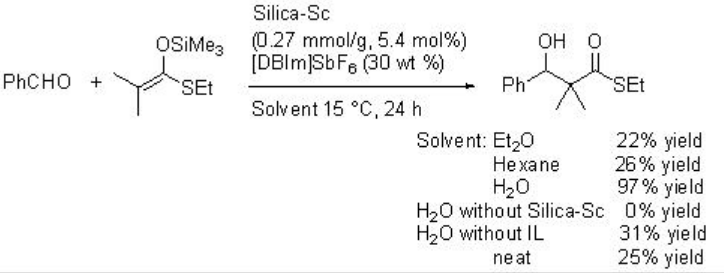
Asymmetric version of the reaction has been subsequently developed employing L-1 as chiral source with moderate enantioselectivity (Scheme \(\PageIndex{12}\)).
Michael Reaction
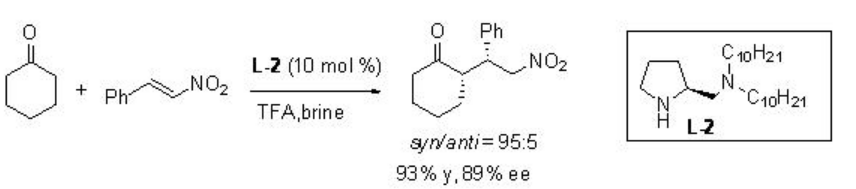
Asymmetric Michael reaction of ketones with β -nitrostyrene has been studied using proline derivative L-2 in brine with good enantioselectivity (Scheme \(\PageIndex{13}\)).
Mannich Reaction
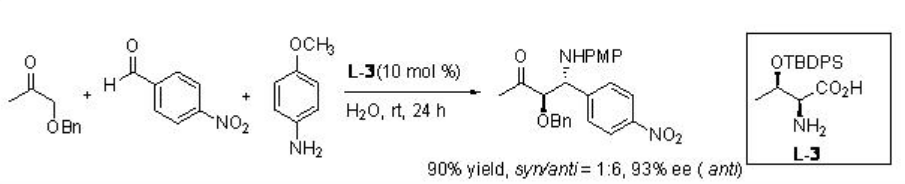
Asymmetirc Mannich reaction of aryl aldehydes, aryl amines and aliphatic ketones occurs in water in the presence of threonine derivative L-3 with excellent diastereoselectivity (Scheme \(\PageIndex{14}\)).
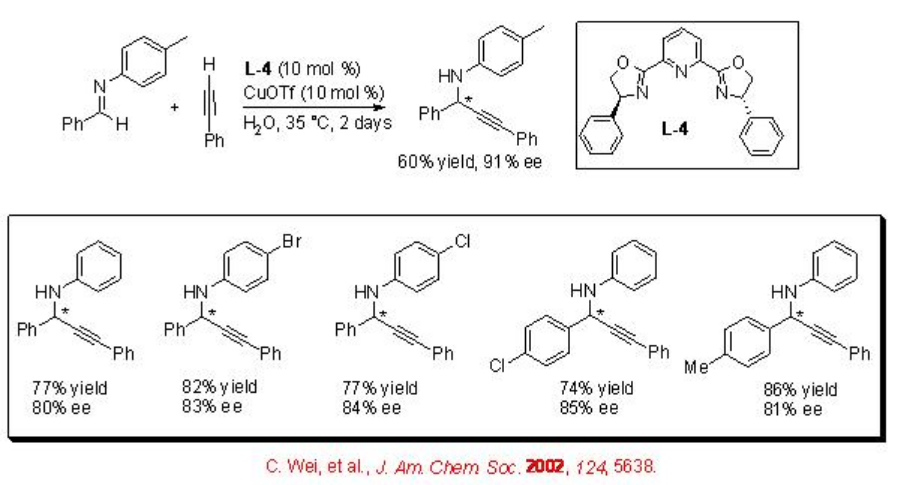
Addition Reactions of Alkynes
Propargylamines are important synthetic intermediates for the synthesis of nitrogen containing compounds in organic synthesis. The direct alkyne-imine addition can be accomplished employing chiral CuOTf- L-4 complex (Scheme \(\PageIndex{15}\)). The method is simple and affords a diverse range of propargylic amines with high enantioselectivity.
Pauson-Khand Reaction
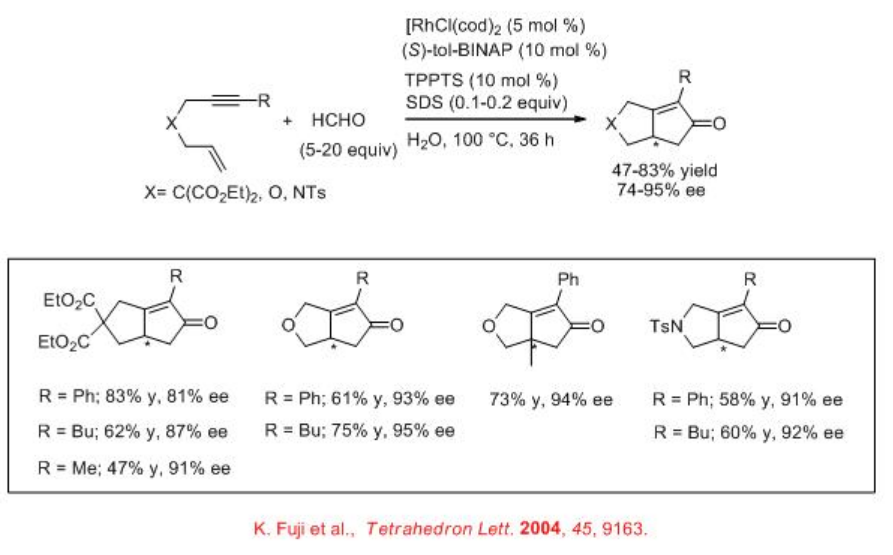
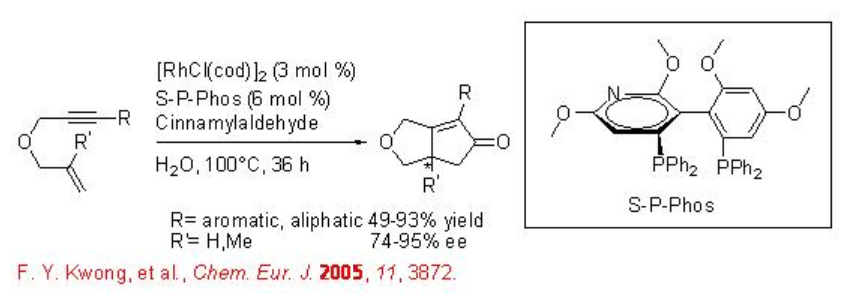
Asymmetric Pauson-Khand reaction can be performed using chiral Rh complexes in water. The complex derived from [RhCl(cod)]2 and (S)-tol-BINAP has been found to be effective for the Pauson-Khand reaction employing HCHO as CO source with good enantioselectivity (Scheme \(\PageIndex{16}\)). Chiral rhodium complex derived from [RhCl(cod)]2 and S-P-Phos has also been used for the Pauson-Khand reaction with similar enantioselectivity (Scheme \(\PageIndex{17}\)).