23.5: Spectroscopic Properties of Amines
- Page ID
- 22340
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Infrared and Ultraviolet Spectra
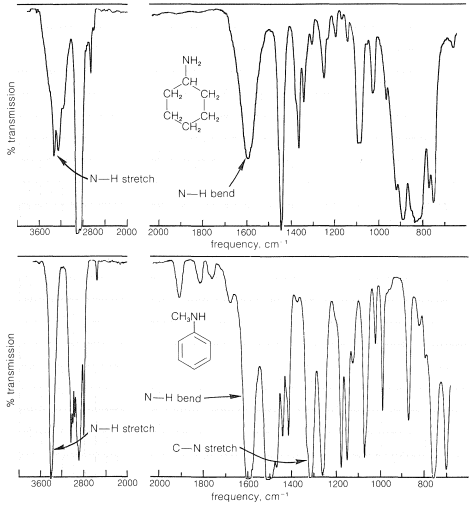
A characteristic feature of the infrared spectra of primary and secondary amines is the moderately weak absorption at \(3500 \: \text{cm}^{-1}\) to \(3300 \: \text{cm}^{-1}\), which corresponds to \(\ce{N-H}\) stretching vibrations. Primary amines have two such bands in this region, whereas secondary amines generally show only one band. Absorption is shifted to lower frequencies by hydrogen bonding, but because \(\ce{NH} \cdots \colon \ce{N}\) bonding is weaker than \(\ce{OH} \cdots \colon \ce{O}\) bonding, the shift is not as great and the bands are not as intense as are the absorption bands of hydrogen-bonded \(\ce{O-H}\) groups (see Table 9-2). Bands corresponding to \(\ce{N-H}\) bending vibrations are observed around \(1600 \: \text{cm}^{-1}\). Absorptions corresponding to \(\ce{C-N}\) vibrations are less easily identifiable, except in the case of arenamines, which absorb fairly strongly near \(1300 \: \text{cm}^{-1}\). Spectra that illustrate these effects are shown in Figure 23-4.
The ultraviolet absorptions of simple saturated amines occur at rather short wavelengths \(\left( \sim 220 \: \text{nm} \right)\) and are not particularly useful for identification. These are \(n \rightarrow \sigma^*\) transitions that correspond to the antibonding \(\sigma\) orbital of a \(\ce{C-N}\) bond.
NMR Spectra
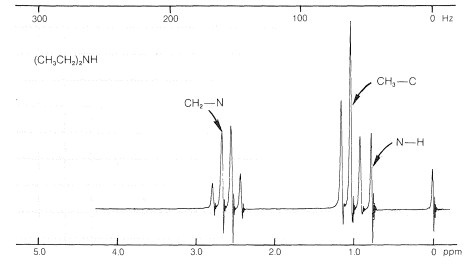
The proton nmr spectra of amines show characteristic absorptions for \(\ce{H-C-N}\) protons around \(2.7 \: \text{ppm}\). The positions of the resonances of \(\ce{N-H}\) protons show considerable variability as the result of differences in degree of hydrogen bonding (Section 9-10E). Sometimes the \(\ce{N-H}\) resonance has nearly the same chemical shift as the resonances of \(\ce{CH_3-C}\) protons (as with \(\ce{N}\)-ethylethanamine, Figure 23-5).
A further complication associated with \(\ce{N-H}\) and \(\ce{H-C-N}\) resonances is their variable chemical shift and line width in the presence of acidic substances because of a chemical exchange process of the type illustrated in Equation 23-1:
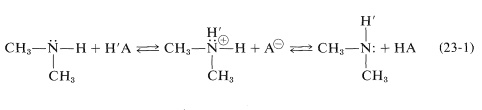
Depending on the rate at which the proton transfers of Equation 23-1 occur and the concentrations of the reactants, the chemical shift of the \(\ce{N-H}\) proton will come between that of pure \(\ce{(CH_3)_2NH}\) and pure \(\ce{HA}\). Except at high acid concentrations, this exchange eliminates any observable coupling between the \(\ce{N-H}\) proton and the \(\ce{N}\)-methyl protons \(\left( \ce{H-C-N-H} \right)\); see Section 9-10I.
In Section 9-10L, we discussed \(\ce{^{13}C}\) nmr and its many applications to structural problems. The nmr of \(\ce{^{15}N}\) nuclei has similar possibilities but, because \(\ce{^{15}N}\) is only \(0.37\%\) of natural nitrogen and has an even smaller nuclear magnetic moment than \(\ce{^{13}C}\), it is very difficult to detect \(\ce{^{15}N}\) resonances at the natural abundance level.\(^2\) Indeed, natural \(\ce{^{15}N}\) has to be observed for about a \(6 \times 10^{10}\) longer time than protons to achieve the same signal-to-noise ratio! Despite this difficulty, natural-abundance \(\ce{^{15}N}\) spectra can be obtained for many compounds (even enzymes) and, in some cases, provide very useful chemical information (see Figure 24-4).
Mass Spectra of Amines
The most prominent cleavage of the parent molecular ion \(\ce{M^+}\) derived from amines occurs at the \(\ce{C}_\beta\)-\(\ce{C}_\alpha\) bond to give an imminium ion which, for ethanamine, has \(m/e = 30\):
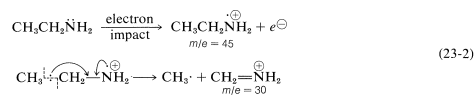
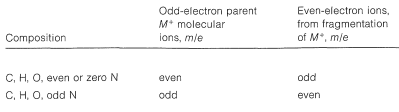
It is helpful in identifying the molecular ion of an organonitrogen compound to remember that the \(m/e\) value of \(\ce{M^+}\) will be an uneven number if the ion contains one or another odd number of nitrogen atoms. Thus ethanamine, \(\ce{C_2H_7N}\), gives an \(\ce{M^+}\) of \(m/e = 45\). For all other elemental compositions of \(\ce{C}\), \(\ce{H}\), \(\ce{O}\), or with an even number of nitrogens, the molecular ion will have an even \(m/e\) value.
The cleavage reaction of Equation 23-2 reveals other useful generalizations. Whatever its source, a parent molecular ion, \(\ce{M^+}\), has one unpaired electron and is properly described as an odd-electron ion (a radical cation). When a parent molecular ion fragments, it does so homolytically, as shown in Equation 23-2, and produces a radical and an ion in which the electrons are paired - an even-electron ion. The \(m/e\) value of an even-electron ion is an even number for any elemental composition of \(\ce{C}\), \(\ce{H}\), \(\ce{O}\) in combination with an odd number of nitrogens. These generalizations are summarized in Table 23-2 and can be useful in the interpretation of mass spectra.
\(^2\)The abundant nitrogen nucleus, \(\ce{^{14}N}\), has a magnetic moment but generally gives very poor nmr spectra with very broad lines. The reason is the \(\ce{^{14}N}\) usually "relaxes" rapidly, which means that its nuclear magnetic states have short lifetimes (see Section 27-1).
Contributors and Attributions
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."


