16.2: The Carbonyl Bond
- Page ID
- 22271
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Comparison with Carbon-Carbon Double Bonds
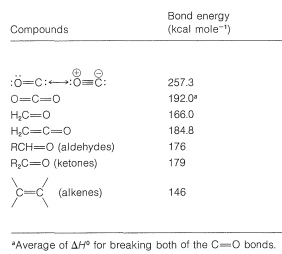
The carbonyl bond is both a strong bond and a reactive bond. The bond energy varies widely with structure, as we can see from the carbonyl bond energies in Table 16-1. Methanal has the weakest bond \(\left( 166 \: \text{kcal} \right)\) and carbon monoxide the strongest \(\left( 237.3 \: \text{kcal} \right)\). Irrespective of these variations, the carbonyl bond not only is significantly stronger but also is more reactive than a carbon-carbon double bond. A typical difference in stability and reactivity is seen in hydration:
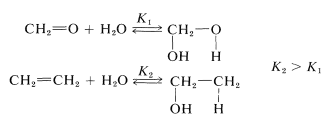
The equilibrium constant for ethene hydration is considerably greater than for methanal hydration, largely because the carbon-carbon double bond is weaker. Even so, methanal adds water rapidly and reversibly at room temperature without need for a catalyst. The corresponding addition of water to ethene occurs only in the presence of strongly acidic catalysis (Section 10-3E, Table 15-2).
Table 16-1: Carbonyl Bond Energies
Structure and Reactivity
The reactivity of the carbonyl bond is primarily due to the difference in electronegativity between carbon and oxygen, which leads to a considerable contribution of the dipolar resonance form with oxygen negative and carbon positive:
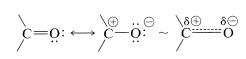
The polarity of the carbonyl bond facilitates addition of water and other polar reagents relative to addition of the same reagent to alkene double bonds. This we have seen previously in the addition of organometallic compounds \(\overset{\delta \ominus}{\ce{R}} \overset{\delta \oplus}{\ce{-MgX}}\) and \(\overset{\delta \ominus}{\ce{R}} \overset{\delta \oplus}{\ce{-Li}}\) to carbonyl compounds (Section 14-12A). Alkene double bonds are normally untouched by these reagents:
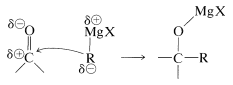
Likewise, alcohols add readily to carbonyl compounds, as described in Section 15-4E. However, we must keep in mind the possibility that, whereas additions to carbonyl groups may be rapid, the equilibrium constants may be small because of the strength of the carbonyl bond.
Further Considerations of Reactivity
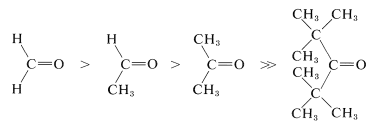
The important reactions of carbonyl groups characteristically involve addition at one step or another. For the reactions of organometallic reagents and alcohols with carbonyl compounds (Chapters 14 and 15), you may recall that steric hindrance plays an important role in determining the ratio between addition and other, competing reactions. Similar effects are observed in a wide variety of other reactions. We expect the reactivity of carbonyl groups in addition processes to be influenced by the size of the substituents thereon, because when addition occurs the substituent groups are pushed back closer to one another. In fact, reactivity and equilibrium constant decrease with increasing bulkiness of substituents, as in the following series (also see Table 15-3):
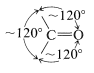
Strain effects also contribute to reactivity of cyclic carbonyl compounds. The normal bond angles around a carbonyl group are about \(120^\text{o}\):
Consequently if the carbonyl group is on a small carbocyclic ring, there will be substantial angle strain and this will amount to about \(120^\text{o} - 60^\text{o} = 60^\text{o}\) of strain for cyclopropanone,
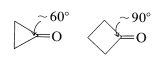
and \(120^\text{o} - 90^\text{o} = 30^\text{o}\) of strain for cyclobutanone (both values being for the \(\angle \ce{C-C-C}\) at the carbonyl group). Addition of a nucleophile such as \(\ce{CH_3OH}\) (cf. Section 15-4E) to these carbonyl bonds creates a tetrahedral center with less strain in the ring bonds to \(\ce{C_1}\):
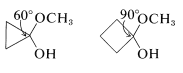
Thus the hemiketal from cyclopropanone will have \(109.5^\text{o} = 60^\text{o} = 49.5^\text{o}\), and that from cyclobutanone \(109.5^\text{o} - 90^\text{o} = 19.5^\text{o}\) of strain at \(\ce{C_1}\). This change in the angle strain means that a sizable enhancement of both the reactivity and equilibrium constant for addition is expected. In practice, the strain effect is so large that cyclopropanone reacts rapidly with methanol to give a stable hemiketal from which the ketone cannot be recovered. Cyclobutanone is less reactive than cyclopropanone, but more reactive than cyclohexanone or cyclopentanone.
Electrical effects also are important in influencing the ease of addition to carbonyl groups. Electron-attracting groups facilitate the addition of nucleophilic reagents to carbon by increasing its positive character:

Thus compounds such as the following add nucleophilic reagents readily:
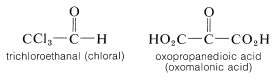
\(^1\)An electrical dipole results when unlike charges are separated. The magnitude of the dipole, its dipole moment, is given by \(e \times r\), where \(e\) is the magnitude of the charges and \(r\) is the distance the charges are separated. Molecular dipole moments are measured in debye units \(\left( \text{D} \right)\). A pair of ions, \(\overset{\oplus}{\ce{C}}\) and \(\overset{\ominus}{\ce{O}}\), as point charges at the \(\ce{C=O}\) distance of \(1.22 \: Å\), would have a dipole moment of \(5.9 \: \text{D}\). Thus, if the dipole moment of a carbonyl compound is \(2.7 \: \text{D}\), we can estimate the "\(\%\) ionic character" of the bond to be \(\left( 2.7/5.9 \right) \times 100 = 46\%\). The analysis is oversimplified in that the charges on the atom are not point charges and we have assumed that all of the ionic character of the molecule is associated with the \(\ce{C=O}\) bond. One should be cautious in interpreting dipole moments in terms of the ionic character of bonds. Carbon dioxide has no dipole moment, but certainly has polar \(\ce{C=O}\) bonds. The problem is that the dipoles associated with the \(\ce{C=O}\) bonds of \(\ce{CO_2}\) are equal and opposite in direction to each other and, as a result, cancel. Thus, \(\overset{\delta \ominus}{\ce{O}} \overset{\delta \oplus}{\ce{-C}} \overset{\delta \ominus}{\ce{-O}}\) has no net dipole moment, even though it has highly polar bonds.
Contributors and Attributions
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."


