15.12: Cyclic Ethers
- Page ID
- 21998
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Nomenclature of Cyclic Ethers
Ring compounds containing nitrogen, oxygen, sulfur, or other elements as ring atoms generally are known as heterocyclic compounds, and the ring atoms other than carbon are the hetero atoms. Over the years, the more common heterocyclic compounds have acquired a hodge-podge of trivial names, such as ethylene oxide, tetrahydrofuran, and dioxane. Systematic naming of ring compounds is necessary but, unfortunately, several competing systems have been developed. We prefer the simplest procedure, which is to name the simple heterocycles as oxa, aza, and thia-derivatives of cycloalkanes. However, this procedure has not been accepted (or adopted) universally and we are obliged to deal with the usages in existing literature. Having lived through at least two cycles of drastic changes in these matters, the authors hope that the simple procedure will prevail in the long run, but the long run is still ahead.
We summarize here the rules of the so-called Hantzsch-Widman nomenclature system for heterocycles, which currently is the fashionable procedure, although relegated to second-class status by a recent, very practical approach to organic nomenclature.\(^1\)
- Ring size is denoted by the stem, ir, et, ol, in, ep, oc, on, or ec for 3-, 4-, 5-, 6-, 7-, 8-, 9-, or 10-membered rings, respectively.
- The kind of hetero atom present is indicated by the prefix, oxa, thia, or aza for oxygen, sulfur, or nitrogen, respectively; the prefixes dioxa, dithia, or diaza denote two oxygen, sulfur, or nitrogen atoms. When two or more different hetero atoms are present, they are cited in order of preference: oxygen before sulfur before nitrogen, as in the prefixes oxaza for one oxygen and one nitrogen, and thiaza for one sulfur and one nitrogen.
- The degree of unsaturation is specified in the suffix. A list of appropriate suffixes and their stems according to ring sizes is given in Table 15-5. Notice that the suffix changes slightly according to whether the ring contains nitrogen.
Table 15-5: Stems, Suffix, and Ring Size of Heterocyclic Compounds

4. Numbering of the ring starts with the hetero atom and proceeds around the ring so as to give substituents (or other hetero atoms) the lowest numbered positions. When two or more different hetero atoms are present, oxygen takes precedence over sulfur and sulfur over nitrogen for the number one position. Examples follow to illustrate both the heterocycloalkane and the Hantzsch-Widman systems. Trivial names also are included.
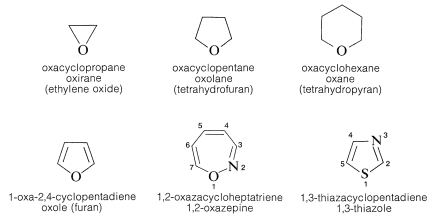
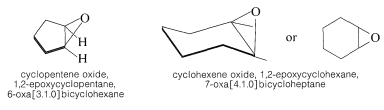
Although Hantzsch-Widman system works satisfactorily (if you can remember the rules) for monocyclic compounds, it is cumbersome for polycyclic compounds. In the case of oxiranes it is simplest for conversational purposes to name them as oxides of the cycloalkenes or epoxy derivatives of the corresponding cycloalkanes. The oxabicycloalkane names seem preferable for indexing purposes, particularly because the word “oxide” is used in many other connections.
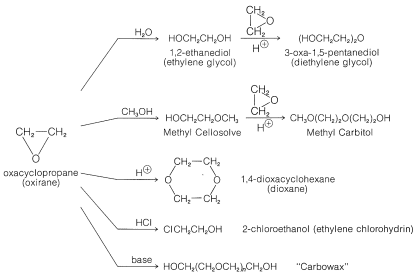
Reactivity of Cyclic Ethers - Oxacyclopropanes (Oxiranes)
Oxacyclopropane (oxirane), the simplest cyclic ether, is an outstanding exception to the generalization that most ethers are resistant to cleavage. Like cyclopropane, the three-membered ring is highly strained and readily opens under mild conditions. Indeed, the importance of oxacyclopropane as an industrial chemical lies in its readiness to form other important compounds. The major products derived from it are shown in Figure 15-5.
The lesser known four-membered cyclic ether, oxacyclobutane (oxetane), \(\ce{(CH_2)_3O}\), also is cleaved readily, but less so than oxacyclopropane. Oxacyclopentane (oxolane, tetrahydrofuran) is a relatively unreactive water-miscible compound with desirable properties as an organic solvent. It often is used in place of diethyl ether in Grignard reactions and reductions with lithium aluminum hydride.
Preparation of Oxacyclopropane
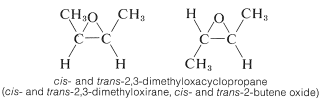
Three-membered cyclic ethers are important as reactive intermediates in organic synthesis. Like the cyclopropanes, the vicinal\(^2\) disubstituted compounds have cis and trans isomers:
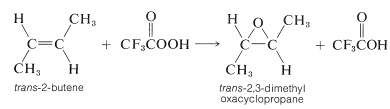
The most important method of preparation involves oxidation, or "epoxidation", of an alkene with a peroxycarboxylic acid, \(\ce{RCO_3H}\). This reaction achieves suprafacial addition of oxygen across the double bond, and is a type of electrophilic addition to alkenes:
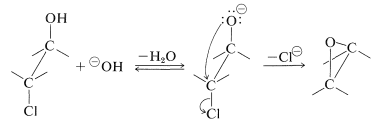
Oxacyclopropanes also can be prepared from vicinal chloro- or bromoalcohols and a base. This is an internal \(S_\text{N}2\) reaction and, if the stereochemistry is correct, proceeds quite rapidly, even if a strained ring is formed:
Ring-Opening Reactions of Oxacyclopropanes
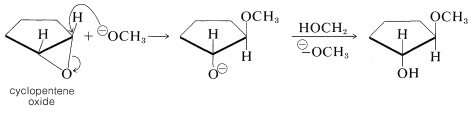
Unlike most ethers, oxacyclopropanes react readily with nucleophilic reagents. These reactions are no different from the nucleophilic displacements previously encountered in Chapter 8, except that the leaving group, which is the oxygen of the oxide ring, remains a part of the original molecule. The stereochemistry is consistent with an \(S_\text{N}2\) mechanism because inversion of configuration at the site of attack occurs. Thus cyclopentene oxide yields products with the trans configuration:
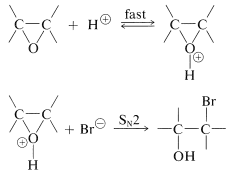
Acidic conditions also can be used for the cleavage of oxacyclopropane rings. An oxonium ion is formed first, which subsequently is attacked by the nucleophile in an \(S_\text{N}2\) displacement or forms a carbocation in an \(S_\text{N}1\) reaction. Evidence for the \(S_\text{N}2\) mechanism, which produces inversion, comes not only from the stereochemistry but also from the fact that the rate is dependent on the concentration of the nucleophile. An example is ring opening with hydrogen bromide:
The same kind of mechanism can operate in the formation of 1,2-diols by acid-catalyzed ring-opening with water as the nucleophile:
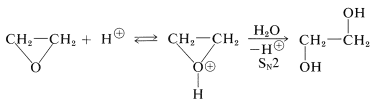
Some acid-catalyzed solvolysis reactions of oxacyclopropanes appear to proceed by \(S_\text{N}1\) mechanisms involving carbocation intermediates. Evidence for the \(S_\text{N}1\) mechanism is available from the reactions of unsymmetrically substituted oxacyclopropanes. For example, we would expect the conjugate acid of 2,2-dimethyloxacyclopropane to be attacked by methanol at the primary carbon by an \(S_\text{N}2\) reaction and at the tertiary carbon by an \(S_\text{N}1\) reaction:
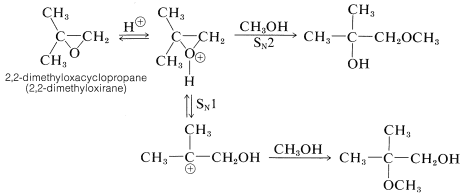
Because both products actually are obtained, we can conclude that both the \(S_\text{N}1\) and \(S_\text{N}2\) mechanisms occur. The \(S_\text{N}1\) product, the tertiary ether, is the major product.
Metal Complexes of Cyclic Polyethers
We have emphasized the contrasts in properties between the ionic compounds such as sodium chloride, and the nonpolar organic compounds, such as the alkanes and arenes. There are great difficulties in dissolving the extreme types of these substances in a mutually compatible medium for carrying on chemical reactions, as, for example, in \(S_\text{N}\) reactions of organic halides with alkali-metal salts (Sections 8-3 and 8-7F). The essence of the problem is that electrostatic forces in ionic crystals of inorganic salts are strong, and nonpolar solvents simply do not have the solvating power for ions to make dissolution of the crystals a favorable process. However, it has long been known that polyethers, such as the "glymes" (Section 15-10), are able to assist in the dissolution of ionic compounds through their ability to solvate metal cations by providing multiple complexing sites:
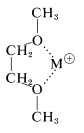
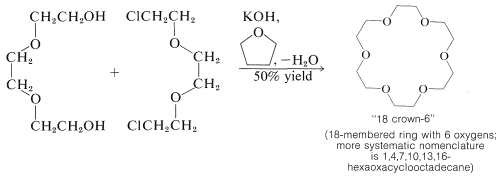
In 1967, C. J. Pedersen reported the synthesis of a series of cyclic polyethers, which he called "crown ethers", that have shown great promise for brining together what traditionally have been regarded as wholly incompatible substances - even achieving measurable solubilities of salts such as \(\ce{NaCl}\), \(\ce{KOH}\), and \(\ce{KMnO_4}\) in benzene. The crown ethers can be regarded as cyclic "glymes" and are available by \(S_\text{N}2\)-type cyclization reactions:
The crown ethers and many modifications of them (especially with nitrogen replacing one or more of the oxygens) function by coordinating with metal cations and converting them into less polar entities that are more stable in solution, even in a nonpolar solvent, than they are in the crystal.
Many of the crown ethers have considerable specificity with regard to the metal with which they complex. Ring size as well as the number and kind of hetero atoms are very important in this connection. 18-Crown-6 is especially effective for potassium:
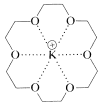
An important application for the crown ethers in synthetic work is for solubilization of salts such as \(\ce{KCN}\) in nonpolar solvents for use in \(S_\text{N}2\) displacements. If the solvent has a low anion-solvating capability, then the reactivity of the anion is enhanced greatly. Consequently many displacement reactions that proceed slowly at elevated temperatures will proceed at useful rates at room temperatures, because the energy of "desolvating" the anion before it undergoes \(S_\text{N}2\) displacement is low (Section 8-7F). For example, potassium fluoride becomes a potent nucleophilic reagent in nonpolar solvents when complexed with 18-crown-6:
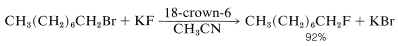
Acetals and Ketals as Ethers
The grouping \(\ce{C-O-C-O-C}\) is characteristic of an acetal or a ketal (see Section 15-4E), but it also can be regarded as an ether with two ether links to one carbon. Compared to other ethers (except for the oxacyclopropanes), substances with the \(\ce{C-O-C-O-C}\) group are very active toward acidic reagents, as pointed out in connection with their formation from alcohols (Section 15-4E) and their sue as protecting groups for the \(\ce{OH}\) function (Section 15-9C).
\(^1\)J. H. Fletcher, O. C. Dermer, and R. B. Fox (Editors), "Nomenclature of Organic Compounds, Principles and Practice." Advances in Chemistry Series, No. 126, American Chemical Society, Washington, D.C., 1974.
\(^2\)Vicinal means substituted on adjacent carbons.
Contributors and Attributions
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."


