13.6: Approaches to Planning Practical Organic Syntheses
- Page ID
- 22065
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Chemistry is unique among the physical and life sciences in one very important respect. It can be manipulated extensively to man’s design. That is, molecular structures can be designed and then constructed by choosing appropriate chemical reactions. This is chemical synthesis, which has been developed to such a degree that the economies and indeed the living standards of the industrialized nations have come to depend on it. Not everyone agrees that the present state of civilization in the industrialized nations is a way station to the millenium. But whether one agrees or not, there is no question that chemical synthesis has played an enormous role in making possible the accessories of modem life.
Chemical synthesis is not a science that can be taught or learned by any well-defined set of rules. Some classify synthesis as more art than science because, as with all really creative endeavors, to be very successful requires great imagination conditioned by a wealth of background knowledge and experience. The problems of synthesis basically are problems in design and planning. Given the objective of synthesizing a specific organic compound, there always is a variety of ways that the objective can be achieved, either from the same or from different starting materials. What we hope to do here is to show how one can go about developing efficient syntheses from available starting materials. However, practice in planning syntheses is imperative to obtaining a good grasp of the principles and problems involved. This will be up to you; no one else can do it for you. Practice also will help greatly to convert short-term memories of organic reactions to longer-term memories through repeated review and demonstrated relevance.
Methodology
In almost all syntheses the target compound is defined precisely, both as to structure and stereochemistry. Regardless of whether the synthesis is destined to be carried out on an industrial scale or on a laboratory scale, careful planning is required. The usual methodology for the planning stage involves two, not wholly independent, steps. First, one considers the various possible ways the desired carbon skeleton can be constructed, either from smaller molecules or by changes in some existing skeleton. Second, means are considered for generation of desired functional groups on the desired carbon skeleton. In many cases, the desired functional groups can be generated as a consequence of the reactions whereby the desired skeleton itself is generated. Alternative syntheses almost always are possible and one should proceed on the notion that the first sequence one thinks of is unlikely to be the best.
The choice of the best route usually is made by considering:
- The availability of the starting materials
- The cost of the starting materials and the equipment needed
- The simplicity of the various steps and the scale of synthesis
- The number of separate steps involved
- The yield in each step
- The ease of separation and purification of the desired product from by-products and stereoisomers
These considerations are dealt with in the following sections and in subsequent chapters.
Starting Materials
Availability of the starting materials obviously is a limiting factor in any synthetic operation. As far as laboratory-type synthesis is concerned, “availability” means that the starting materials either may be bought “off the shelf” or may be prepared easily by standard methods from other inexpensive and available compounds. For large-scale industrial syntheses, the limiting factor usually is the cost of the starting materials, including the energy required.
But in some cases the limiting factor may be problems in disposal of the byproducts. Costs will vary according to geographical location and will fluctuate widely, as with crude oil costs, so as to cause obsolescence and constant change in the chemical industry. However, it is worth remembering that the cheapest organic starting materials available are methane, ethene, ethyne, propene, butenes, benzene, and methylbenzene (toluene). Any chemical that can be prepared easily in high yield from one of these hydrocarbons is likely to be relatively inexpensive, readily available, and useful as a starting material in more involved syntheses.
The Yield Problem
Among the factors considered in choosing among several possible synthetic routes is: Which gives the best yield? The definition of yield and its distinction from another useful term, conversion, should be clearly understood. To help you understand, consider a specific example, the bromination of 2-methyl-propane to give tert-butyl bromide as the desired product. This type of reaction is carried on best with an excess of hydrocarbon to avoid polysubstitution (Section 4-5), and if we use such an excess of hydrocarbon, bromine will be the limiting reagent. This means simply that the amount of the desired product that could be formed is determined, or limited, by the amount of bromine used:


Suppose we start with one mole of hydrocarbon and 0.2 mole of bromine and, after a specified reaction time, 0.1 mole of bromine has reacted. If only the desired product were formed, and there were no other losses of hydrocarbon or bromine,
 \[\% \: \text{conversion} = \frac{\text{moles of limiting reagent reacted}}{\text{moles of limiting reagent initially present}} = \frac{0.1}{0.2} \times 100 = 50\%\]
\[\% \: \text{conversion} = \frac{\text{moles of limiting reagent reacted}}{\text{moles of limiting reagent initially present}} = \frac{0.1}{0.2} \times 100 = 50\%\]
If there are no losses in isolating the product or in recovering unused starting material, then
 \[\% \: \text{yield} = \frac{\text{moles of product}}{\text{moles of limiting reagent initially present}} = \frac{0.1}{0.1} \times 100 = 100\%\]
\[\% \: \text{yield} = \frac{\text{moles of product}}{\text{moles of limiting reagent initially present}} = \frac{0.1}{0.1} \times 100 = 100\%\]
Now suppose all of the 0.2 mole of bromine reacts, 0.08 mole of the desired product can be isolated, and 0.7 mole of hydrocarbon is recovered. Under these circumstances, the percent conversion is \(100\%\), because all of the bromine has reacted. The yield can be figured in different ways depending on which starting material one wishes to base the yield. Based on bromine (which would be logical because bromine is the more expensive reagent) the yield of tert-butyl bromide is \(\left( 0.08/0.2 \right) \times 100 = 40\%\). However, one also could base the yield of tert-butyl bromide on the unrecovered hydrocarbon, and this would be \(\left[ 0.08/\left( 1.0-0.7 \right) \right] \times 100 = 27\%\).
In a multistep synthesis, the overall percent yield is the product of the fractional yields in each step times 100 and decreases rapidly with the number of steps. For this reason, a low-yield step along the way can mean practical failure for the overall sequence. Usually, the best sequence will be the one with the fewest steps. Exceptions arise when the desired product is obtained as a component of a mixture that is difficult to separate. For example, one could prepare 2-chloro-2-methylbutane in one step by direct chlorination of 2-methyl-butane (Section 4-5A). But because the desired product is very difficult to separate from the other, isomeric monochlorinated products, it is desirable to use a longer sequence that may give a lower yield but avoids the separation problem. Similar separation problems would be encountered in a synthesis that gives a mixture of stereoisomers when only one isomer is desired. Again, the optimal synthesis may involve a longer sequence that would be stereospecific for the desired isomer.
One way of maximizing the yield is to minimize the number of sequential steps and, whenever possible, to use parallel rather than sequential reactions. For example, suppose that we wish to synthesize a compound \(\ce{ABCDEF}\) by linking together \(\ce{A}\), \(\ce{B}\), \(\ce{C}\), \(\ce{D}\), \(\ce{E}\), and \(\ce{F}\). The sequential approach would involve at least five steps as follows:
 \[\ce{A} \overset{\ce{B}}{\rightarrow} \ce{AB} \overset{\ce{C}}{\rightarrow} \ce{ABC} \overset{\ce{D}}{\rightarrow} \ce{ABCD} \overset{\ce{E}}{\rightarrow} \ce{ABCDE} \overset{\ce{F}}{\rightarrow} \ce{ABCDEF}\]
\[\ce{A} \overset{\ce{B}}{\rightarrow} \ce{AB} \overset{\ce{C}}{\rightarrow} \ce{ABC} \overset{\ce{D}}{\rightarrow} \ce{ABCD} \overset{\ce{E}}{\rightarrow} \ce{ABCDE} \overset{\ce{F}}{\rightarrow} \ce{ABCDEF}\]
If each of these steps proceeds in \(90\%\) yield, the overall yield would be \(\left( 0.90 \right)^5 \times 100 = 59\%\).
One possible parallel approach would involve synthesis of the fragments \(\ce{ABC}\) and \(\ce{DEF}\) followed by the combination of these to \(\ce{ABCDEF}\):

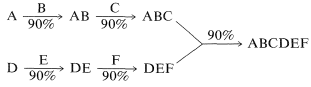
There are still at least five reaction steps, but only three sequential steps; and if each of these proceeds in \(90\%\) yield, the overall yield would be \(\left( 0.90 \right)^3 \times 100 = 73\%\). The parallel approach is especially important in the synthesis of polymeric substances such as peptides, proteins, and nucleic acids in which many subunits have to be linked.
Finally, product yields are very dependent on manipulative losses incurred in each step by isolating and purifying the synthetic intermediates. The need to minimize losses of this kind is critically important in very lengthy syntheses.
Contributors and Attributions
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."


