14.2: Acids- Properties and Examples
- Page ID
- 47554
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Examine properties of acids.
Many people enjoy drinking coffee. A cup first thing in the morning helps start the day. But keeping the coffee maker clean can be a problem. Lime deposits build up after a while and slow down the brewing process. The best cure for this is to put vinegar (dilute acetic acid) in the pot and run it through the brewing cycle. The vinegar dissolves the deposits and cleans the maker, which will speed up the brewing process back to its original rate. Just be sure to run water through the brewing process after the vinegar, or you will get some really horrible coffee.
Acids
Acids are very common in some of the foods that we eat. Citrus fruits such as oranges and lemons contain citric acid and ascorbic acid, which is better known as vitamin C. Carbonated sodas contain phosphoric acid. Vinegar contains acetic acid. Your own stomach utilizes hydrochloric acid to digest food. Acids are a distinct class of compounds because of the properties of their aqueous solutions as outlined below:
- Aqueous solutions of acids are electrolytes, meaning that they conduct electrical current. Some acids are strong electrolytes because they ionize completely in water, yielding a great many ions. Other acids are weak electrolytes that exist primarily in a non-ionized form when dissolved in water.
- Acids have a sour taste. Lemons, vinegar, and sour candies all contain acids.
- Acids change the color of certain acid-base indicates. Two common indicators are litmus and phenolphthalein. Blue litmus turns red in the presence of an acid, while phenolphthalein turns colorless.
- Acids react with active metals to yield hydrogen gas. Recall that an activity series is a list of metals in descending order of reactivity. Metals that are above hydrogen in the activity series will replace the hydrogen from an acid in a single-replacement reaction, as shown below:
\[\ce{Zn} \left( s \right) + \ce{H_2SO_4} \left( aq \right) \rightarrow \ce{ZnSO_4} \left( aq \right) + \ce{H_2} \left( g \right) \label{eq1} \] - Acids react with bases to produce a salt compound and water. When equal moles of an acid and a base are combined, the acid is neutralized by the base. The products of this reaction are an ionic compound, which is labeled as a salt, and water.
It should not be hard for you to name several common acids (but you might find that listing bases is a little more difficult). Below is a partial list of some common acids, along with some chemical formulas:
|
Chemist Name
|
Common Name | Uses |
|---|---|---|
| hydrochloric acid, HCl | muriatic acid (used in pools) and stomach acid is HCl | Used in cleaning (refining) metals, in maintenance of swimming pools, and for household cleaning. |
| sulfuric acid, H2SO4 | Used in car batteries, and in the manufacture of fertilizers. | |
| nitric acid, HNO3 | Used in the manufacture of fertilizers, explosives and in extraction of gold. | |
| acetic acid, HC2H3O2 | vinegar | Main ingredient in vinegar. |
| carbonic acid, H2CO3 | responsible for the "fizz" in carbonated drinks | As an ingredient in carbonated drinks. |
| citric acid, C6H8O7 | Used in food and dietary supplements. Also added as an acidulant in creams, gels, liquids, and lotions. | |
| acetylsalicylic acid, C6H4(OCOCH3)CO2H | aspirin | The active ingredient in aspirin. |
What exactly makes an acid an acid, and what makes a base act as a base? Take a look at the formulas given in the above table and take a guess.
Hydrochloric Acid
Hydrochloric acid is a corrosive, strong mineral acid with many industrial uses. A colorless, highly pungent solution of hydrogen chloride (HCl) in water. Hydrochloric acid is usually prepared by treating \(\ce{HCl}\) with water.
\[ \ce{\displaystyle HCl (g) + H2O (l) \longrightarrow H_3O^{+}(aq) + Cl^{-} (aq) } \nonumber \]
Hydrochloric acid can therefore be used to prepare chloride salts. Hydrochloric acid is a strong acid, since it is completely dissociated in water. Hydrochloric acid is the preferred acid in titration for determining the amount of bases.
Sulfuric Acid
Sulfuric acid is a highly corrosive strong mineral acid with the molecular formula \(\ce{H2SO4}\). Sulfuric acid is a diprotic acid and has a wide range of applications including use in domestic acidic drain cleaners,[as an electrolyte in lead-acid batteries, and in various cleaning agents. It is also a central substance in the chemical industry.

Because the hydration of sulfuric acid is thermodynamically favorable (and is highly exothermic) and the affinity of it for water is sufficiently strong, sulfuric acid is an excellent dehydrating agent. Concentrated sulfuric acid has a very powerful dehydrating property, removing water (\(\ce{H2O}\)) from other compounds including sugar and other carbohydrates and producing carbon, heat, steam. Sulfuric acid behaves as a typical acid in its reaction with most metals by generating hydrogen gas (Equation \ref{Eq1}).
\[\ce{M + H2SO4 → M(SO4) + H2 } \label{Eq1} \]
Nitric Acid
Nitric acid (\(\ce{HNO3}\)) is a highly corrosive mineral acid and is also commonly used as a strong oxidizing agent. Nitric acid is normally considered to be a strong acid at ambient temperatures. Nitric acid can be made by reacting nitrogen dioxide (\(\ce{NO_2(g)}\)) with water.
\[\ce{3 NO2(g) + H2O (l)→ 2 HNO3 (ag) + NO(g)} \nonumber \]
Nitric acid reacts with most metals, but the details depend on the concentration of the acid and the nature of the metal. Dilute nitric acid behaves as a typical acid in its reaction with most metals (e.g., nitric acid with magnesium, manganese or zinc will liberate \(\ce{H2}\) gas):
\[\ce{Mg + 2 HNO3 → Mg(NO3)2 + H2 } \nonumber \]
\[\ce{Mn + 2 HNO3 → Mn(NO3)2 + H2 } \nonumber \]
\[\ce{Zn + 2 HNO3 → Zn(NO3)2 + H2 } \nonumber \]
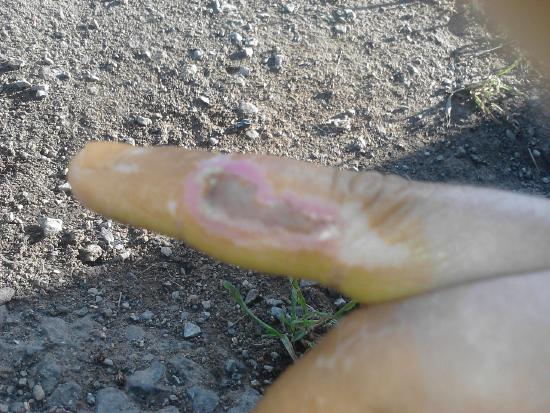
Nitric acid is a corrosive acid and a powerful oxidizing agent. The major hazard it poses is chemical burn, as it carries out acid hydrolysis with proteins (amide) and fats (ester) which consequently decomposes living tissue (Figure \(\PageIndex{2}\)). Concentrated nitric acid stains human skin yellow due to its reaction with the keratin
Carbonic Acid
Carbonic acid is a chemical compound with the chemical formula \(\ce{H2CO3}\) and is also a name sometimes given to solutions of carbon dioxide in water (carbonated water), because such solutions contain small amounts of \(\ce{H2CO3(aq)}\). Carbonic acid, which is a weak acid, forms two kinds of salts: the carbonates and the bicarbonates. In geology, carbonic acid causes limestone to dissolve, producing calcium bicarbonate—which leads to many limestone features such as stalactites and stalagmites. Carbonic acid is a polyprotic acid, specifically it is diprotic, meaning that it has two protons which may dissociate from the parent molecule.
When carbon dioxide dissolves in water, it exists in chemical equilibrium (discussed in Chapter 15), producing carbonic acid:
\[\ce{CO2 + H2O <=> H2CO3} \nonumber \]
The reaction can be pushed to favor the reactants to generate \(\ce{CO2(g)}\) from solution, which is key to the bubbles observed in carbonated beverages (Figure \(\PageIndex{3}\)).

Formic Acid
Formic acid (\(\ce{HCO2H}\)) is the simplest carboxylic acid and is an important intermediate in chemical synthesis and occurs naturally, most notably in some ants. The word "formic" comes from the Latin word for ant, formica, referring to its early isolation by the distillation of ant bodies. Formic acid occurs widely in nature as its conjugate base formate.
Citric Acid
Citric acid (\(\ce{C6H8O7}\)) is a weak organic tricarboxylic acid that occurs naturally in citrus fruits. The citrate ion is an intermediate in the TCA cycle (Krebs cycle), a central metabolic pathway for animals, plants and bacteria. Because it is one of the stronger edible acids, the dominant use of citric acid is used as a flavoring and preservative in food and beverages, especially soft drinks.

Acetylsalicylic Acid
Acetylsalicylic acid (also known as aspirin) is a medication used to treat pain, fever, and inflammation. Aspirin, in the form of leaves from the willow tree, has been used for its health effects for at least 2,400 years.
Aspirin is a white, crystalline, weakly acidic substance.
Summary
A brief summary of key aspects of several acids commonly encountered by students was given. Acids are a distinct class of compounds because of the properties of their aqueous solutions.
Contributions & Attributions
Peggy Lawson (Oxbow Prairie Heights School). Funded by Saskatchewan Educational Technology Consortium.

