9.7: Degradation of amino acids
- Page ID
- 433998
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Understand the catabolism of amino acids, including transamination, oxidative amination, and urea cycle that takes care of the \(\ce{N}\) and processing of the \(\ce{C}\) skeleton of the amino acid to intermediates that enter into citric acid cycle for energy production.
Amino acids are the products of stage 1 of protein catabolism. Usually, amino acids are used to synthesize proteins and other substances that need nitrogen, e.g., nucleotides. Only about 10% of our energy is usually derived from amino acids. Still, when there is a shortage of carbohydrates and fats, e.g., during fasting or starvation, amino acids are used as an alternate energy source. Prolonged use of amino acids as an energy source may lead to the destruction of essential tissues.
Degradation of amino acids has two sets of catabolic pathways: one that deals with the processing of \(\ce{N}\) and the other that deals with the processing of the remaining \(\ce{C}\) skeleton of the amino acids.
Processing of \(\ce{N}\) of amino acids
Processing of \(\ce{N}\) usually happens in the liver and has three major stages: i) transamination, ii) oxidative deamination, and iii) urea cycle, as illustrated with some examples in Figure \(\PageIndex{1}\).

Transamination
Transamination is the process of exchange of an ammonium (\(\ce{-NH3^{+}}\)) group of amino acids with ketone (\(\ce{C=O}\)) group of an \(\alpha\)-ketoacid. Usually \(\alpha\)-amino acids exchange \(\ce{-NH3^{+}}\) group with ketone (\(\ce{C=O}\)) group of an \(\alpha\)-ketoglutarate. For example, alanine exchanges its \(\ce{-NH3^{+}}\) group with a \(\ce{C=O}\) of an \(\alpha\)-ketoglutarate producing a new \(\alpha\)-keto acid (pyruvate in this example) and a new \(\alpha\)-amino acids (L-glutamate in this example), as shown in reaction 1 in Figure \(\PageIndex{1}\). Other \(\alpha\)-keto acids can also receive \(\ce{-NH3^{+}}\) of amino acids also. For example, L-glutamate can transfer its \(\ce{-NH3^{+}}\) to oxaloacetate that regenerates \(\alpha\)-ketoglutarate and produces aspartate, as shown in reaction 3 in Figure \(\PageIndex{1}\).
Oxidative deamination
Oxidative deamination reaction replaces \(\ce{-NH3^{+}}\) with a \(\ce{C=O}\), producing an \(\alpha\)-keto acid and an ammonium (\(\ce{NH4^{+}}\)) ion, as shown in reaction 2 in Figure \(\PageIndex{1}\). This is an oxidation reaction that is coupled with the reduction of \(\ce{NAD^{+}}\) to \(\ce{NADH}\). It happens in mitochondria where \(\ce{NADH}\) enters oxidative phosphorylation pathway to produce \(\ce{ATP's}\). The other product, i.e., \(\ce{NH4^{+}}\), is a toxic substance that needs to be disposed off. Some of \(\ce{NH4^{+}}\) may be used in anabolic reactions, e.g., reactions 4 and 5 in Figure \(\PageIndex{1}\), but most of it enters the urea cycle.
Urea cycle
Ammonium ion \(\ce{NH4^{+}}\) is a toxic substance that is converted to less toxic urea in the urea cycle by the following overall reaction.
\(\ce{2NH4^{+} + CO2 -> \underbrace{H2N-\!\!{\overset{\overset{\huge\enspace\!{O}}|\!\!|\enspace}{C}}\!\!-NH2}_{Urea} + 2H^{+} + H2O}\).
Before entry into the urea cycle, \(\ce{NH4^{+}}\) reacts with two \(\ce{ATP's}\) and bicarbonate (\(\ce{HCO3^{-}}\)), producing carbamoyl phosphate, two \(\ce{ADP's}\) and phosphate (\(\ce{P_i}\), as shown below.

This reaction is catalyzed by an enzyme carbamoyl phosphate synthetase I. Blood contains \(\ce{HCO3^{-}}\) which is the product of dissolution of \(\ce{CO2}\). The product of the above reaction, i.e., Carbamoyl phosphate, goes through four reactions called the urea cycle illustrated in Figure \(\PageIndex{2}\).

- The first reaction of the urea cycle is catalyzed by ornithine transcarbamylase. The \(\ce{-COO^{-}}\) group of ornithine substitutes phosphate (\(\ce{P_{i}}\) from carbamoyl phosphate producing citrulline in mitochondria. Citrulline moves out from the matrix into the cytoplasm.
 The second reaction is a condensation reaction between the carbonyl (\(\ce{C=O}\)) group of citrulline and \(\ce{-NH3^{+}}\) group of aspartate that produces argininosuccinate. This reaction takes place in the cytosol and is catalyzed by argininosuccinate synthetase.
The second reaction is a condensation reaction between the carbonyl (\(\ce{C=O}\)) group of citrulline and \(\ce{-NH3^{+}}\) group of aspartate that produces argininosuccinate. This reaction takes place in the cytosol and is catalyzed by argininosuccinate synthetase.- In the third reaction, argininosuccinate is cleaved by the enzyme argininosuccinase to form arginine, which stays in the cycle, and fumarate, which leaves the cycle.
- In the fourth reaction, arginase cleaves arginine to form urea and ornithine. Ornithine is transported back to the matrix in mitochondria to start the next urea cycle, and urea is released into the blood.
Since \(\ce{HCO3^{-}}\) is produced by the dissolution of \(\ce{CO2}\) in water, the \(\ce{NH4^{+}}\) + \(\ce{HCO3^{-}}\) are equivalent to \(\ce{NH3}\) + \(\ce{CO2}\) + \(\ce{H2O}\). So, the overall equation of the urea cycle becomes:
\(\ce{NH3 + CO2 + H2O + aspartate + 3ATP + 3H2O -> urea + fumarate + 2ADP + 2P_{i} + AMP + PP_{i} + H2O}\)
Since \(\ce{NH3}\) is removed to convert aspartate into fumarate along with \(\ce{PP_{i} + H2O -> 2P_{i}}\), substituting these for aspartate and fumarate simplifies the above equation into:
\(\ce{2NH3 + CO2 + 3ATP + 3H2O -> urea + 2ADP + 4P_{i} + AMP}\)
One \(\ce{NADH}\) is produced during oxidative deamination of L-glutamate (reaction 2 in Figure \(\PageIndex{1}\)). Another \(\ce{NADH}\) is produced when fumarate from reaction 3 of the urea cycle is processed in the citric acid cycle, i.e., fumarate is converted into malate by enzyme fumarase and then malate is oxidized to oxaloacetate at the expense of reduction of \(\ce{NAD^{+}}\) to \(\ce{NADH}\) by enzyme malate dehydrogenase. Adding these reactions to the above reaction of the urea cycle results in the following overall reaction.
\(\ce{CO2 + glutamate + aspartate + 3ATP + 2NAD^{+} + 3H2O -> urea + \alpha{-ketoglutarate} + oxaloacetate + 2ADP + 2P_{i} + AMP + 2PP_{i} + 2NADH}\)
Three \(\ce{ATP}\) are consumed, one of them to \(\ce{AMP}\) that makes total equivalent to four \(\ce{ATP's}\) consumed but two \(\ce{NADH}\) enter oxidative phosphorylation and yield ~5\(\ce{ATP}\), i.e., there is net production of an \(\ce{ATP}\) in the process of \(\ce{N}\) of an amino acid.
The urea released into the blood is later filtered out by the kidneys and excreted with urine. An adult passes about 25 to 30 g of urea in urine per day. If urea is not eliminated properly, it builds to toxic levels and needs medical treatment, like dialysis. Reducing protein intake also lowers urea output.
The link between the urea cycle and the citric acid cycle
The urea cycle and citric acid cycle are two separate cycles but are linked with each other. Aspartate that provides one \(\ce{N}\) in the urea cycle is produced by oxaloacetate transamination, which is an intermediate in the citric acid cycle. Fumarate is a product of the urea cycle that is an intermediate of the citric acid cycle and returns to it.
processing of \(\ce{C}\) skeleton of amino acids
\(\alpha\)-Ketoacids are left as \(\ce{C}\) skeleton of amino acid after transamination or oxidative deamination. For example, pyruvate is left after transamination of alanine and \(\alpha\)-ketoglutarate is left after oxidative deamination of glutamate, as shown in reactions 1 and 2, respectively, in Figure \(\PageIndex{1}\). \(\alpha\)-Ketoacids are either used as precursors for the synthesis of other compounds, or they enter the citric acid cycle to produce \(\ce{CO2}\), \(\ce{H2O}\), and energy. \(\alpha\)-Ketoglutarate and oxaloacetate are \(\alpha\)-keto acids that are intermediates in the citric acid cycle and directly enter the cycle. Other \(\alpha\)-keto acids go through a series of reactions to convert into one of the intermediates in the citric acid cycle, or they convert to pyruvate or acetyl-(\ce{CoA}\) that enter the citric acid cycle, as illustrated in Figure \(\PageIndex{3}\). Details of the conversation of \(\alpha\)-keto acids into intermediates of the citric acid cycle or pyruvate or acetyl-(\ce{CoA}\) are not described here.
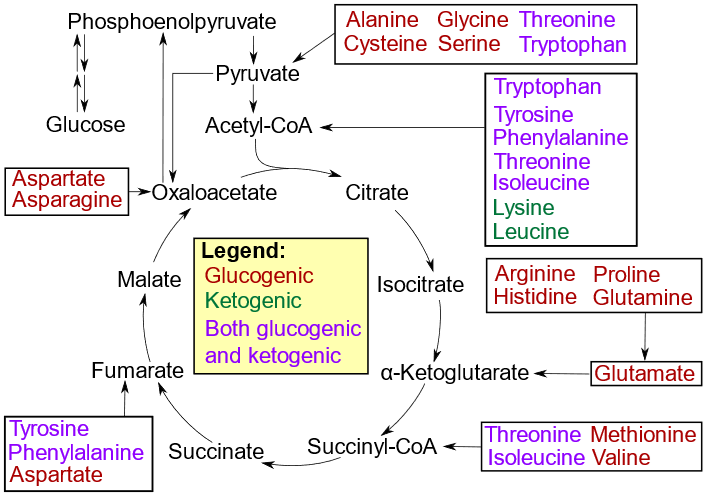
Amino acids that can degrade to pyruvate or oxaloacetate are called glucogenic because these products can form glucose through the glucogenesis pathway. For example, alanine yields pyruvate, and aspartate yield oxaloacetate, as shown in reactions 1 and 3 in Figure \(\PageIndex{1}\). Amino acids that degrade to acetyl-(\ce{CoA}\) or acetoacetic acid, which can not form glucose but can be converted into ketone bodies, are called ketogenic. Lysine and leucine are ketogenic amino acids. Several amino acids can catabolize to produce both glucogenic and ketogenic intermediates, and they fall into both classes, as shown in Figure \(\PageIndex{3}\).


