9.6: Oxidation of fatty acids
- Page ID
- 433997
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Understand the catabolism of fatty acids, i.e., \(\beta\)-oxidation of fatty acids, the reactions involved in the process, the number of cycles needed, and the calculation of ATP yield per fatty acid.
- Understand obesity, ketogenesis, ketosis, ketoacidosis, and catabolism of glycerol, associated with \(\beta\)-oxidation of fatty acids.
\(\beta\)-Oxidation of fatty acids
Although glucose is a quick energy source that animals need, fatty acids have higher energy per unit mass. Therefore, fats are the main energy storage compounds in animals. Fatty acids (\(\ce{R-COOH}\)) in fats are a major source of energy for animals. Hydrolysis of fats, i.e., the 1st stage of catabolism of fats, begins in the digestive tract and completes in the cytosol of the cells. The second stage of fatty acid catabolism starts with the conversion of (\(\ce{R-COOH}\)) to the thioester group of coenzyme A, (\(\ce{R-CO-S-CoA}\)), i.e., an acyl-\(\ce{CoA}\), at the cost of energy of \(\ce{ATP}\) to \(\ce{AMP}\) conversion. The thioester is called an activated fatty acid. Some of the important terms used in this section are illustrated in the figure below.

Acyl-\(\ce{CoA}\) is transported from the cytosol into mitochondria for the second stage of its catabolism. In the second stage, the Acyl-\(\ce{CoA}\) is fragmented into two \(\ce{C's}\) fragments in the form of acetyl-\(\ce{CoA}\). The activated fatty acid goes through a set of four reactions in which the \(\beta\ce{C}\) is oxidized to a carbonyl \(\ce{C=O}\) group. Another coenzyme A makes thioester group with the \(\beta\)-\(\ce{C=O}\), and an acetyl-\(\ce{CoA}\) group is split off, leaving behind an acyl-\(\ce{CoA}\) with two \(\ce{C's}\) less than the initial acyl-\(\ce{CoA}\). This process is called \(\beta\)-Oxidation of fatty acids. The process repeats on the fragment acyl-\(\ce{CoA}\) again and again until the last acyl-\(\ce{CoA}\) fragment left is acetyl-\(\ce{CoA}\). The acetyl-\(\ce{CoA}\) enters the citric acid cycle, which is the third stage of the catabolism, as in the case of the catabolism of glucose. These reactions are explained next.
Activation of fatty acids
The activation of a fatty acid begins with an SN2 reaction of acylate anion as a nucleophile with \(\ce{ATP}\). It produces acyl-adenylate and pyrophosphate, as shown below.

Pyrophosphate is removed by hydrolysis, making the reaction irreversible: \(\ce{PP_i + H2O -> 2P_i + 2H^{+}}\).
Acyl-adenylate goes through a nucleophilic acyl substitution reaction with coenzyme A (\(\ce{HS-CoA}\)) attacking as a nucleophile and AMP as leaving group.
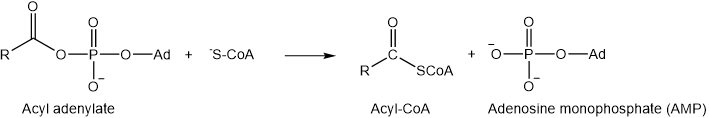
As described next, acyl-CoA product is transported from the cytosol into mitochondria for the \(\beta\)-oxidation process.
Reaction 1 -oxidation
Reaction 1 is the dehydrogenation of of acyl-\(\ce{CoA}\) by enzyme acyl-\(\ce{CoA}\)-dehydrogenase that removes \(\ce{H}\) from \(\alpha\) and \(\beta\) \(\ce{C's}\) generating a trans \(\ce{C=C}\) bond. An \(\ce{FAD}\) is reduced to \(\ce{FADH2}\) as the acyl-\(\ce{CoA}\) is oxidized in this reaction. Resonance stabilization of the \(\ce{C=C}\) bond by conjugated \(\ce{C=O}\) group makes the product stable, lowering the activation energy for the reaction.

Reaction 2 -hydration
The \(\ce{C=C}\) is hydrated by addition of \(\ce{H2O}\) that selectively installs an \(\ce{-OH}\) group at \(\beta\)\(\ce{C}\), creating a new chiral center in L-configuration. This is an electrophilic addition reaction. The \(\ce{C=O}\) makes the \(\beta\)\(\ce{C}\) more nucleophilic by withdrawing electrons from it by resonance. That is why the \(\beta\)\(\ce{C}\) selectively reacts with the incoming \(\ce{H2O}\) electrophile.

Reaction 3 -oxidation
The secondary \(\ce{-OH}\) group is oxidized to a ketone (\(\ce{C=O}\)) group at the expense of reduction of \(\ce{NAD^{+}}\) to \(\ce{NADH}\) by the following reaction.

Reaction 4 -cleavage by thiolysis
The fourth reaction is a nucleophilic acyl substitution reaction in which an anion of coenzyme A (\(\ce{^{-}S-CoA}\)) acts as a nucleophile, the ketone (\(\ce{C=O}\)) as electrophile and \(\ce{^{-}CH2-CO-S-CoA}\) as the leaving group. \(\ce{^{-}CH2-CO-S-CoA}\) is a good leaving group because the negative charge is resonance stabilized and mainly resides on \(\ce{O}\) of carbonyl (\(\ce{C=O}\)) group. A proton later neutralizes the negative charge.

The second product of the above reaction is an acyl-\(\ce{CoA}\) with two \(\ce{2C's}\) less than the initial acyl-\(\ce{CoA}\).
Number of \(\beta\)-oxidation cycles
The product acyl-\(\ce{CoA}\) of the first \(\beta\)-oxidation cycle goes through the process of four reactions again and again until the last acyl-\(\ce{CoA}\) product is acetyl-\(\ce{CoA}\), i.e., \(\ce{CH3CO-S-COA}\). The process is illustrated in Figure \(\PageIndex{1}\), with the help of an example of \(\beta\)-oxidation of stearic acid which is a typical \(\ce{18C's}\) saturated fatty acid.

If the starting fatty acid has \(\ce{nC's}\), there are: \(\frac{n}{2}\) acetyl-\(\ce{CoA}\) produced. Since the last cycle produces two acetyl-\(\ce{CoA}\), the number of cycles for a fatty acid containing \(\ce{nC's}\) is: \(\frac{n}{2}-1\). For example, stearic acid has \(\ce{18C's}\) and it goes through: \(\frac{18}{2}-1 = 8~\beta\text{-oxidation cycles}\).
\(\ce{ATP}\) Yield from fatty acid oxidation
Each \(\beta\)-oxidation cycle of a fatty acid yields one \(\ce{FADH2}\), one \(\ce{NADH}\) and one acetyl-\(\ce{CoA}\). These molecules produce \(\ce{ATP's}\) when they enter the citric acid cycle and oxidative phosphorylation, as described earlier in the catabolism of glucose. One \(\ce{FADH2}\) ≈ 1.5 \(\ce{ATP's}\), one \(\ce{NADH}\) ≈ 2.5 \(\ce{ATP's}\), and one acetyl-\(\ce{CoA}\) ≈ 10 \(\ce{ATP's}\) that makes 14 \(\ce{ATP's}\) per \(\beta\)-oxidation cycle. Since there are (\(\frac{n}{2}-1\)) or (\(0.5\times{n}-1\)) cycles for a fatty acid containing \(\ce{nC's}\), there are: (\((0.5\times{n}-1)\times{14}))~\ce{ATP's}\) produced. The last cycle yields two acetyl-\(\ce{CoA}\), so there are addition \(\ce{10ATP's}\) from the the last acetyl-\(\ce{CoA}\). The activation step converts one \(\ce{ATP}\) to one \(\ce{AMP}\) which is equivalent to conversion of \(\ce{2ATP}\) to \(\ce{2ADP}\). So, after adding ten from the last acetyl-\(\ce{CoA}\) to the formula ( \((0.5\times{n}-1)\times{14})~\ce{ATP's}\) and subtracting 2 for the \(\ce{2ATP's}\) consumed in the activation step, the formula for the number of \(\ce{ATP's}\) produced per fatty acid containing \(\ce{nC's}\) become the following:
\(\ce{ATP's}~\text{produced per fatty acid containing}~\ce{nC's} = ((0.5\times{n}-1)\times{14} + 10 -2)~\ce{ATP's}\).
It simplifies to:
\(\ce{ATP's}~\text{produced per fatty acid containing}~\ce{nC's} = (7\times{n}-6)~\ce{ATP's}\).
For example, stearic acid contains \(\ce{18C's}\) and produces \((7\times18-6) = 120~\ce{ATP's}\). So, one mole of stearic acid (284.48 g/mol) produces 120 mol \(\ce{ATP's}\), which is significantly higher than 32 mol \(\ce{ATP's}\) produced by one mole of glucose (180.156 g/mol). When converted to moles of \(\ce{ATP's}\) produced per unit mass, stearic acid produces 0.42 mol \(\ce{ATP's}\)/g, which is more than two times higher than 0.18 mol \(\ce{ATP's}\)/g of glucose. This is because the average oxidation state of glucose \(\ce{C's}\) is higher than those of fatty acid \(\ce{C's}\). Glucose is a quick energy source, but animals use fats as relatively longer-term energy storage molecules due to their higher energy density.
Unsaturated fatty acids have \(\ce{C=C}\) bond in their alkyl chain. \(\beta\)-Oxidation of unsaturated fatty acids happens the same way as for saturated fatty acids except for the following changes. If \(\ce{C=C}\) bond is a trans-\(\ce{C=C}\) bond between \(\alpha\) and \(\beta\) \(\ce{C'}\), reaction 1 in the \(\beta\)-oxidation is not needed and \(\ce{FADH2}\) from reaction 1 is not produced. If \(\ce{C=C}\) bond is a cis-\(\ce{C=C}\) bond or it is between \(\beta\) and \(\gamma\) \(\ce{C'}\), It goes through isomerization reactions catalyzed by isomerase enzymes to convert the \(\ce{C=C}\) bond into a trans \(\ce{C=C}\) bond between \(\alpha\) and \(\beta\) \(\ce{C'}\) before reaction 3 happens on it. Unsaturated fatty acids yield a little less \(\ce{ATP's}\) due to one \(\ce{FADH2}\) less produced pre \(\ce{C=C}\) bond, but the difference is small and the formula: \((7\times{n}-6)~\ce{ATP's}\), still gives a reasonably accurate estimate of \(\ce{ATP}\) yield.



 Although carbohydrates and glucose are quick energy sources, fat has more energy per mass or volume. Storage of fats for long-term energy supply is an important survival feature for several animals. For example, hibernating animals like polar bear converts food into fats during summer when food is plenty and utilize stored fats to survive for months without food during hibernation in winter. Whales and penguins are kept warm by a layer of body fat called blubber and use it as an energy source to survive when food is unavailable. Camel store fat in their hump and can survive for months without food and water by utilizing the fat reserves. Migrator birds also store fat and use it as an energy source during migration.
Although carbohydrates and glucose are quick energy sources, fat has more energy per mass or volume. Storage of fats for long-term energy supply is an important survival feature for several animals. For example, hibernating animals like polar bear converts food into fats during summer when food is plenty and utilize stored fats to survive for months without food during hibernation in winter. Whales and penguins are kept warm by a layer of body fat called blubber and use it as an energy source to survive when food is unavailable. Camel store fat in their hump and can survive for months without food and water by utilizing the fat reserves. Migrator birds also store fat and use it as an energy source during migration.
Humans can store fats. In earlier times, major human food was vegetables, and fats in food accounted for about 20% of food calories, but these days more than 60% of food calories are from fats. The cumulation of too much fat is associated with overweight and obesity. Obesity is an excess accumulation of body fat that negatively affects health. It is measured in terms of body mass index (BMI), which is the ratio of a person's mass to the square of a person's height. BMI 18.5 kg/m2 to 24.9 kg/m2 is normal, less is underweight, more is overweight, and over 30 kg/m2 is obese, as shown in Figure \(\PageIndex{2}\). Obesity is associated with medical conditions like diabetes, high blood pressure, stroke, heart disease, gallstones, arthritis, and some cancers. According to NIH, nearly 3 out of 4 adults aged 20 or above in the US are overweight or obese. It is a preventable condition, as described in the following video message from NIH.

Like insulin regulates blood glucose, leptin is a hormone adipose tissues produce. Leptin governs the long-term balance between food intake and energy expenditure. When there is more adipose tissue (fatty tissues), more leptin is released, which decreases hunger by giving a feeling of fullness, and less fatty tissue means less fat and less leptin, which has the opposite effect. Like diabetes disturbs the function of insulin, obesity may be related to the malfunctioning of leptin. This is an active area of research these days for finding obesity treatment.
Ketone bodies
 Acetyl-\(\ce{CoA}\) produced during the \(\beta\)-oxidation of fatty acids enter the citric acid cycle. When many fatty acids degrade, the citric acid cycle can not take all of the acetyl-\(\ce{CoA}\). The excess acetyl-\(\ce{CoA}\) accumulates in the liver and converts into ketone bodies by a pathway known as ketogenesis, as shown in the figure on the right (Copyright; Sav vas, CC0, via Wikimedia Commons). In this figure, the enzymes are colored red, and the substrates/products are colored blue. The three ketone bodies, i.e., acetoacetate, acetone, and \(\beta\)-hydroxybutyrate, are marked within orange boxes.
Acetyl-\(\ce{CoA}\) produced during the \(\beta\)-oxidation of fatty acids enter the citric acid cycle. When many fatty acids degrade, the citric acid cycle can not take all of the acetyl-\(\ce{CoA}\). The excess acetyl-\(\ce{CoA}\) accumulates in the liver and converts into ketone bodies by a pathway known as ketogenesis, as shown in the figure on the right (Copyright; Sav vas, CC0, via Wikimedia Commons). In this figure, the enzymes are colored red, and the substrates/products are colored blue. The three ketone bodies, i.e., acetoacetate, acetone, and \(\beta\)-hydroxybutyrate, are marked within orange boxes.
Ketone bodies are transported from the liver to other tissues where acetoacetate and \(\beta\)-hydroxybutyrate are converted back to acetyl-\(\ce{CoA}\) and enter the citric acid cycle to produce energy. Normally there is constant production and consumption of acetone bodies maintaining ~1 mg/dL concentration in blood.
Acetone cannot be converted back into acetyl-\(\ce{CoA}\) except in the liver, where it can be converted into lactate and then to pyruvate.
Ketosis
 When the synthesis rate of ketone bodies exceeds their consumption rate, they start accumulating and may start excreting in urine and via breathing -a condition called ketosis. Since two of the three ketone bodies are acids, their accumulation in the blood lowers the blood pH -a condition called ketoacidosis. The smell of acetone, fruity or like nail polish remover smaller, is detectable in the breath of persons suffering from ketosis. Ketone bodies can be tested in blood, urine, or breath, e.g., by using test strips with a color chart to read the results, as shown in the figure on the left. In ketosis, the ketone bodies in the blood are between 0.5 to 3.0 millimole/L (mmol/L). In ketoacidosis, the concentration may go above 10 mmol/L.
When the synthesis rate of ketone bodies exceeds their consumption rate, they start accumulating and may start excreting in urine and via breathing -a condition called ketosis. Since two of the three ketone bodies are acids, their accumulation in the blood lowers the blood pH -a condition called ketoacidosis. The smell of acetone, fruity or like nail polish remover smaller, is detectable in the breath of persons suffering from ketosis. Ketone bodies can be tested in blood, urine, or breath, e.g., by using test strips with a color chart to read the results, as shown in the figure on the left. In ketosis, the ketone bodies in the blood are between 0.5 to 3.0 millimole/L (mmol/L). In ketoacidosis, the concentration may go above 10 mmol/L.
Ketosis can happen during fasting, starvation, prolonged low carbohydrate diets, prolonged intense exercises, alcoholism, or due to uncontrolled diabetes.
 The liver and pancreas play important roles in maintaining blood glucose levels, illustrated in the figure on the right (copyright: C. Muessig, CC BY-SA 3.0, via Wikimedia Commons) and described next. The normal glucose level in the blood is 4.5 to 5.5 mM. When glucose level is above normal, usually after a meal, the pancreas senses it and secrets insulin that increases the flow of glucose into muscles and fatty tissues, where it is converted into glycogen, lowering blood glucose. When the blood glucose level is low, the pancreas secretes another hormone, glucagon, into the bloodstream. Glucagon triggers glycogen breakdown, particularly in the liver, releasing glucose into the bloodstream.
The liver and pancreas play important roles in maintaining blood glucose levels, illustrated in the figure on the right (copyright: C. Muessig, CC BY-SA 3.0, via Wikimedia Commons) and described next. The normal glucose level in the blood is 4.5 to 5.5 mM. When glucose level is above normal, usually after a meal, the pancreas senses it and secrets insulin that increases the flow of glucose into muscles and fatty tissues, where it is converted into glycogen, lowering blood glucose. When the blood glucose level is low, the pancreas secretes another hormone, glucagon, into the bloodstream. Glucagon triggers glycogen breakdown, particularly in the liver, releasing glucose into the bloodstream.
In diabetes, there is either insufficient insulin or not functioning properly. Less glucose is sent to muscles and, as a result, muscles cause \(\beta\)-oxidation of fatty acids to meet the energy needs. Higher levels of acetyl-\(\ce{CoA}\) from the \(\beta\)-oxidation produce more ketone bodies than their consumption, accumulating ketone bodies. It causes ketosis or ketoacidosis. Low pH of blood plasma due to ketoacidosis triggers kidneys to excrete urine with high acid levels. Glucose and ketone bodies also spill into the urine. High glucose concentration in the blood causes higher osmotic pressure, increasing dehydration. The symptoms include frequent urination and excessive thirst. These conditions can be treated with low carbohydrate diets, insulin therapy, or other medications to control diabetes.
Catabolism of glycerol
Glycerol, a part of triglycerides (fats), accounts for about 5% of their energy. Glycerol is first converted into glycerol-3-phosphate by an SN2 reaction of a primary alcohol group of glycerol acting as a nucleophile, phosphorus in the phosphate of ATP as a nucleophile, and ADP as a leaving group. Then, the secondary alcohol of glycerol is oxidized to a ketone group at the expense of reduction of a \(\ce{NAD^{+}}\) coenzyme into \(\ce{NADH}\) as shown below.

Dihydroxyacetone phosphate produced by the second reaction is an intermediate in the glycolysis of glucose and enters the same catabolic pathway.


