7.4: Secondary structure of proteins
- Page ID
- 425776
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Define the secondary structure of proteins and understand the structural features of major secondary structures, including \(\alpha\)-helix, \(\beta\)-pleated sheet, random coil, and triple helix structures.
The polypeptide backbone is composed of repeated \(\ce{-C-C-N{-}}\) units where the side chain (\(\ce{-R}\)) is hanging on the first \(\ce{C}\). The second carbon is the carbonyl (\(\ce{C=O}\)) group and the nitrogen is the amine (\(\ce{-NH{-}}\)) group in amide. The polymer chain acquires confirmation to achieve:
- maximize hydrogen bonding between the \(\ce{C=O}\) and \(\ce{-NH{-}}\) of the same chain (intramolecular) or among the neighboring chains (intermolecular), and
- minimize the steric-strain due to the \(\ce{-R}\) groups.
This way, the polymer backbone acquires repetitive patterns in portions of the backbone.
The secondary structure of proteins comprises organized regions of polypeptide backbone stabilized by hydrogen bonds between atoms.
The two common secondary structures encountered in proteins are (\(\alpha\)-helix and \(\beta\)-pleated sheet. The other portions of the polymer backbone that are regular but not repetitive are called random coils. Triple-helix is another common secondary structure found in collagen proteins in connective tissues. These secondary structures are illustrated in Figure \(\PageIndex{1}\). below and described in the following sections.



\(\alpha\)-Helix
 In the \(\alpha\)-helix portion, the polypeptide chain acquires a coil shape spiraling clockwise from N-terminus to C-terminus, held by hydrogen bonds between the carbonly groups and amine groups in the backbone and side chains protruding outwards, as illustrated in the model shown on the left
In the \(\alpha\)-helix portion, the polypeptide chain acquires a coil shape spiraling clockwise from N-terminus to C-terminus, held by hydrogen bonds between the carbonly groups and amine groups in the backbone and side chains protruding outwards, as illustrated in the model shown on the left
Each \(\ce{N-H}\) is hydrogen bonded with a \(\ce{C=O}\) group four units away, shown by hashed lines in the model. Each side chain (\(\ce{-R}\)) group on an amino acid reside is protruding outwards from the helix.
The \(\alpha\)-helix is represented by a right-handed spiral shape ribbon, as shown by the red-color ribbon in the structure of glucagon shown in Figure \(\PageIndex{2}\). The side chain on each amino acid can be seen protruding out from the helix. The random coil portion of glucagon is shown by a blue-color tube. Since the side chains hang out in free space, short and long-side chain amino acids can easily be accommodated in an \(\alpha\)-helix without steric strain. Keratin, a fibrous protein of hair, fingernails, horns, and wool, is composed of a major portion of \(\alpha\)-helix.

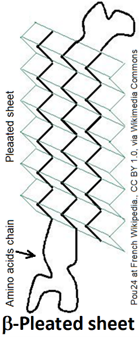
\(\beta\)-Pleated sheet
 The \(\beta\)-Pleated sheet is a portion of polypeptide chains in which sections of polypeptide chains are aligned parallel or antiparallel and held together by hydrogen bonds between the carbonyl groups and amine groups of the neighboring polypeptide chains. The \(\beta\)-Pleated sheet is usually represented as a ribbon with an arrowhead pointing toward the N-terminus, as shown on the right.
The \(\beta\)-Pleated sheet is a portion of polypeptide chains in which sections of polypeptide chains are aligned parallel or antiparallel and held together by hydrogen bonds between the carbonyl groups and amine groups of the neighboring polypeptide chains. The \(\beta\)-Pleated sheet is usually represented as a ribbon with an arrowhead pointing toward the N-terminus, as shown on the right.
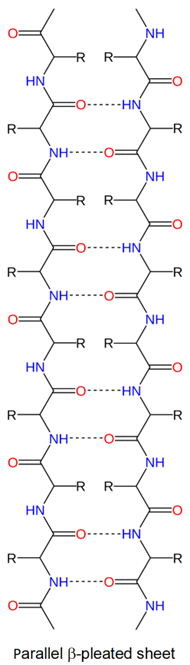
 Since the polypeptide backbone is in a zig-zag shape, multiple chains running parallel to each other in a \(\beta\)-pleated sheet is a pleated shape as shown in Figure \(\PageIndex{3}\). The two neighboring chains are parallel if both have N-terminus on the same side, as shown on the right, and antiparallel if the two adjacent chains have N-terminus on the opposite sides, as shown on the left. The neighboring chains may be different polypeptides or the same polypeptide chain with hairclip-like bents at the ends.
Since the polypeptide backbone is in a zig-zag shape, multiple chains running parallel to each other in a \(\beta\)-pleated sheet is a pleated shape as shown in Figure \(\PageIndex{3}\). The two neighboring chains are parallel if both have N-terminus on the same side, as shown on the right, and antiparallel if the two adjacent chains have N-terminus on the opposite sides, as shown on the left. The neighboring chains may be different polypeptides or the same polypeptide chain with hairclip-like bents at the ends.

The alternate \(\ce{C=O}\) and \(\ce{N-H}\) on the backbone of neighboring chains point towards each other and hydrogen bond with each other, as shown in Figure \(\PageIndex{3}\). The other set of alternate \(\ce{C=O}\) and \(\ce{N-H}\) point to the other side and establish hydrogen bonds with the chains on the other sides. These hydrogen bonds give mechanical strength to the proteins. Silk is made of a fibrous protein fibroin with a significant portion as \(\beta\)-pleated sheets.
The alternate side chains (\(\ce{-R}\)) point above and below in a \(\beta\)-pleated sheet. The amino acids in \(\beta\)-pleated sheets usually have short side chains, such as glycine, valine, alanine, and serine, as the long chains cause more steric strain.
Random coil
Most of the proteins have a mix of \(\alpha\)-helix and \(\beta\)-pleated sheets and organized but not repeated structures between the two that are called random coils as illustrated in Figure \(\PageIndex{4}\). The random coins are represented by tubes.

Triple helix
The triple helix is a secondary structure found in collagen. Collagen is a structural protein that is strong and elastic, present in connective tissues of the tendon, cartilage, blood vessels, skin, and bone. It is the most abundant protein of vertebrates making up 30% to 35% of their proteins by weight.
The triple helix is made of three collagen peptides which are left-handed helices that are placed together on a common axis and displaced (translated) along the axis, forming a right-handed triple helix, as shown in Figure \(\PageIndex{1}\) and Figure \(\PageIndex{5}\).

The collagen peptide is composed of repeat units of Gly-X-Y, where X is usually proline, and Y is generally hydroxyproline - a proline with a hydroxyl (\(\ce{-OH}\) group installed on the side chain. Proline has a secondary \(\alpha\)-amine group that causes bent in the peptide chain, which is needed for the helical structure of collagen peptides. The extra (\(\ce{-OH}\)) in the side chain of hydroxyproline allows more hydrogen bonding in the collagen peptide, which makes connective tissues stronger.


