6.7: Sterols
- Page ID
- 432917
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Understand the core structure of steroids and sterols.
- Understand the structural features of cholesterol, its production, consumption, transport, and medical problems.
- Understand bile acids' structure features, their functions, and medical problems.
- Understand the structural features of steroid hormones, functions, abuses, and medical problems.
What are steroids?
Steroids are biologically active compounds that have a core structure composed of four fused rings in a specific configuration: three six-membered rings designated A, B, and C and one five-membered ring D, as Figure \(\PageIndex{1}\) right. Gonane has this core structure of steroids. Other steroids have gonane skeletons with some groups attached. For example, cholestane has methyl groups at positions 10 and 13 and an aliphatic chain at position 17. Cholesterol has the structure of cholestane. It is a biomarker found in rocks and petroleum deposits.
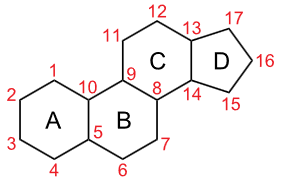 Gonane or sterane with \(\ce{C's}\) numbered
Gonane or sterane with \(\ce{C's}\) numbered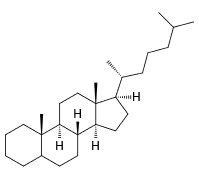 Cholestan with stereochemistry shown
Cholestan with stereochemistry shown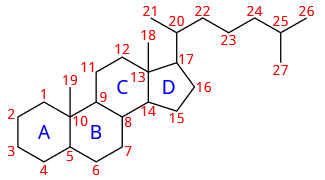 cholestan with \(\ce{C's}\) numbered
cholestan with \(\ce{C's}\) numberedWhat are sterols?
 Sterols are organic compounds derived from gonane with \(\ce{H}\) #3 replaced with an alcohol (\(\ce{-OH}\)) group. The sterols are a sub-class of steroids. The simplest sterol is the alcohol gonane shown in the figure on the right. Other sterols have other groups attached to the gonane structure.
Sterols are organic compounds derived from gonane with \(\ce{H}\) #3 replaced with an alcohol (\(\ce{-OH}\)) group. The sterols are a sub-class of steroids. The simplest sterol is the alcohol gonane shown in the figure on the right. Other sterols have other groups attached to the gonane structure.
Cholesterol
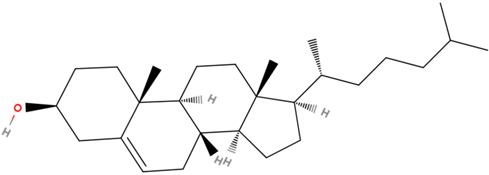
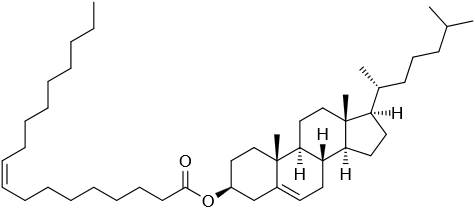
Cholesterol is the most abundant sterol found in animals. It is a structural component of animal cell membranes, a precursor for steroid hormones, bile acids, and vitamin D. Cholesterol has the \(\ce{C}\) skeleton of cholestane, with an \(\ce{-OH}\) group at position #3 and a \(\ce{C=C}\) bond at position 5 and 6, as shown in Figure \(\PageIndex{2}\).

Cholesterol sources and consumption
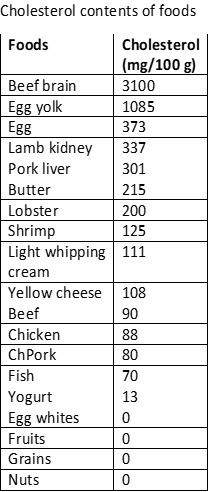 Cholesterol is biosynthesized from lanosterol, which, in turn, is synthesized from squalene, as described in the previous section. Cholesterol is synthesized in the liver using fats, proteins, and carbohydrates as raw materials. An average human weighing 68 kg synthesizes about 1000 mg of cholesterol daily. Food is also a source of cholesterol intake, as shown in the figure on the right. Typically about 307 mg of cholesterol intake is through food per day in the US. The liver reduces cholesterol production to adjust for the intake from food sources.
Cholesterol is biosynthesized from lanosterol, which, in turn, is synthesized from squalene, as described in the previous section. Cholesterol is synthesized in the liver using fats, proteins, and carbohydrates as raw materials. An average human weighing 68 kg synthesizes about 1000 mg of cholesterol daily. Food is also a source of cholesterol intake, as shown in the figure on the right. Typically about 307 mg of cholesterol intake is through food per day in the US. The liver reduces cholesterol production to adjust for the intake from food sources.
Cholesterol is amphiphilic like phospholipids and sphingolipids, with \(\ce{-OH}\) as a polar head and cholestane structure as a hydrophobic tail. The cholesterol molecule is stiff due to restricted movements around \(\ce{C-C}\) bonds locked in the cyclic structure.
Cholesterol is used in the cell membrane to impart rigidity to the ring -the higher the proportion of cholesterol in the cell membrane, the more rigid the membrane.
Usually, cholesterol is 30% of the cell membrane. It is involved in signaling and is found in the brain, plasma membrane, and myelin sheath of nerve cells. Cholesterol is a precursor to vitamin D and steroid hormones, including corticosteroids, such as cortisol and aldosterone, and sex hormones, such as progesterone, estrogens, and testosterone.
The liver recycles the excess cholesterol by converting it into bile acids stored in the gallbladder and excreted into the digestive tract. Bile acids emulsify fats in the food, which helps digest the food. About 50% of the excreted cholesterol is re-absorbed into the bloodstream by the small intestine.
Transport of cholesterol
Cholesterol is insoluble in water like other lipids. It is transported by the blood, which is water rich medium. Like soap carries greases in water during washing, lipoprotein transport glycerolipids and cholesterol in the body. The lipoproteins exist as particles suspended in blood. Glycerides and cholesteryl esters are in the core, and phospholipids, proteins (apolipoprotein), and cholesterol form the outer layer having their hydrophobic parts oriented outwards in contact with water, as illustrated in Figure \(\PageIndex{3}\).
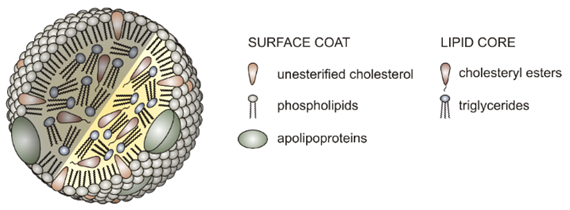
 Lipoproteins are classified based on their density, as shown in the figure on the right (Copyright: Modified from İnfoCan at Turkish Wikipedia., FAL, via Wikimedia Commons, Public domain). Their density is based on the proportion of proteins in them -the higher the proportion of proteins, the higher the density. They also have roles in the transportation process of cholesterol and lipids:
Lipoproteins are classified based on their density, as shown in the figure on the right (Copyright: Modified from İnfoCan at Turkish Wikipedia., FAL, via Wikimedia Commons, Public domain). Their density is based on the proportion of proteins in them -the higher the proportion of proteins, the higher the density. They also have roles in the transportation process of cholesterol and lipids:
- Chylomicrons – Transport triglycerides from the small intestine to the liver.
- Very low-density lipoproteins (VLDL) - transport newly synthesized triglycerides from the liver to the fatty tissues.
- Intermediate-density lipoproteins (IDL) are between VLDL and LDL in density. They are not usually seen in the blood.
- Low-density lipoproteins (LDL) - carry cholesterol from the liver to other tissues. They are also sometimes referred to as "bad cholesterol."
- High-density lipoproteins (HDL) - collect cholesterol from other tissues and return it to the liver. They are sometimes referred to as "good cholesterol."
Excessive fats and cholesterol in the body are associated with diabetes; cancers of the breast, pancreas, and colon; heart attack; and stroke. Lipoproteins transport cholesterol and fats in the body. LDLs transport cholesterol from the liver to the cells where they are needed, and HDLs transport surplus cholesterol back to the liver, where it is converted to other steroids and bile acids. If the LDL level is too high, some cholesterol is deposited in the artery as plagues, as shown in Figure \(\PageIndex{4}\). That is why LDLs are sometimes called bad cholesterol. The plaque causes inflammation and death of the tissue in that area and restricts blood flow. It may lead to a heart attack if the blood flow to the heart is inadequate or a stroke if the blood flow to the brain is insufficient.
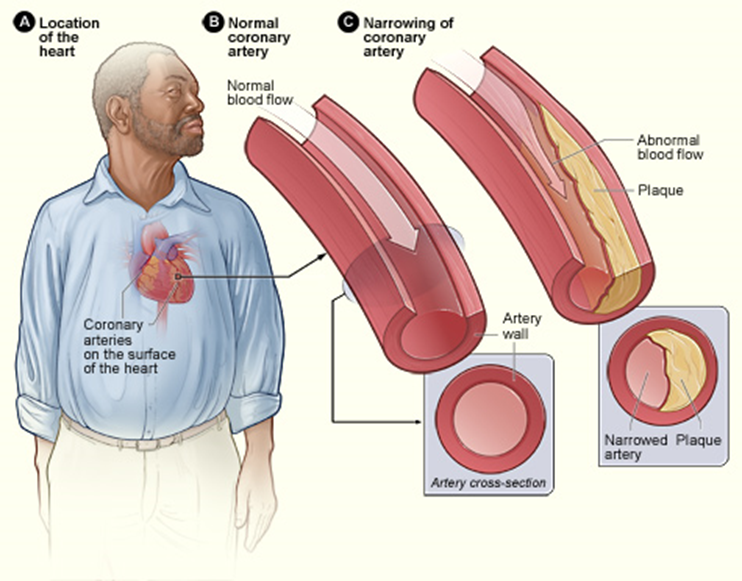
Since HDLs transport the surplus cholesterol back to the liver for recycling and avoid plaque formation, HDLs are also called good cholesterol. The HDL levels must be sufficiently high to prevent a heart attack. A lipid panel test is prescribed to assess the risk of a heart attack. It measures the levels of total cholesterol, LDLs, HDLs, and triglycerides in the blood. The normal levels are the following.
| Total cholesterol | About 150 mg/dL, less is better |
| LDL ("bad") cholesterol | About 100 mg/dL, less is better |
| HDL ("good") cholesterol | At least 40 mg/dL in men and 50 mg/dL in women; higher is better |
| Triglycerides | Less than 150 mg/dL, less is better |
Bile salts
Bile salts are synthesized from cholesterol in the liver. First, additional \(\ce{-OH}\) groups are incorporated, and the alkyl side chain is shortened and oxidized to a carboxylic acid (\(\ce{-COOH}\)) group. Then the \(\ce{-COOH}\) group is esterified with either glycine or taurine that imparts a negative charge group, as illustrated in Figure \(\PageIndex{5}\).
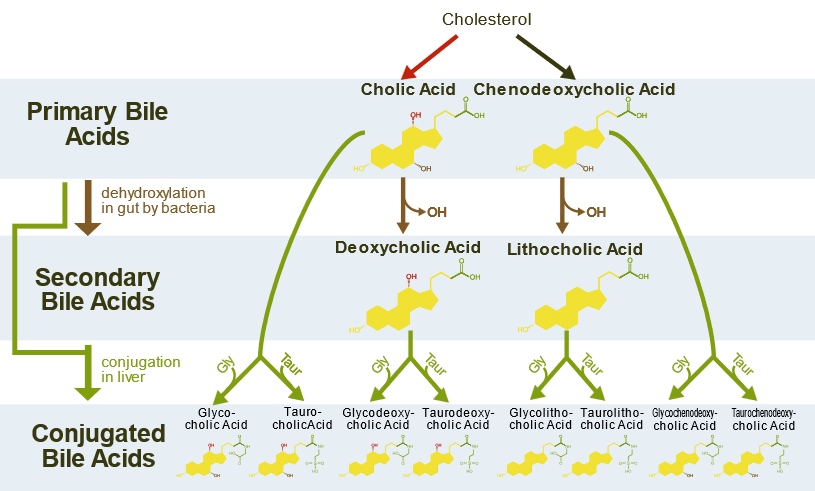
Bile salts act like soaps with an ionic head and the remaining hydrophobic part. They are stored in the gallbladder and released into the small intestine, where they emulsify fats, i.e., convert the larger fat drops into smaller droplets, just like greases and dirt are emulsified by soaps. The smaller droplets have a larger surface area, increasing the fats' reaction rate with the pancreatic lipase enzymes, as illustrated in Figure \(\PageIndex{6}\).

Since bile salts are excreted with the feces, they remove cholesterol in two ways: 1) they are themselves the products of cholesterol, and 2) they dissolve cholesterol in the form of bile salt-cholesterol particles that are eliminated.
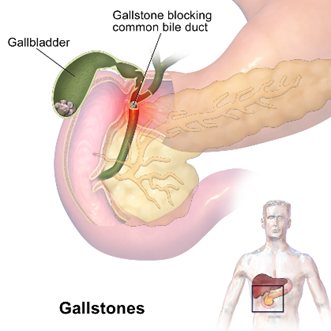
When a large amount of cholesterol accumulates in the gallbladder, it turns into solid particles, i.e., gallstones. The gallstones are almost 100% cholesterol with a small amount of calcium salts, glycerophospholipids, and fatty acids. Small gallstones pass through the bile duct and enter the duodenum, i.e., the initial part of the small intestine, and are excreted. However, large gallstones may get stuck in the bile duct and cause pain, as illustrated in Figure \(\PageIndex{7}\). If a gallstone block the bile duct, the bile can not be excreted. Then the bile pigment, known as bilirubin, will back up into the liver and be passed via blood. It causes jaundice which gives a yellow color to the skin and whiteness to the eyes.
Steroid hormones
Steroid hormones have structural similarities with cholesterol and are derived from it. Two significant subclasses of steroid hormones are sex and adrenocortical hormones, described in the following sections.
Sex hormones
The male sex hormones are called androgens which include testosterone and androsterone. The female sex hormones are estrogens, e.g., estradiol and estrone, and progestins, e.g., progesterone. These five of the most essential sex hormones are shown in the figure below. All five are present in males and females, but androgens are produced more in males, and estrogens and progestins are produced more in females.
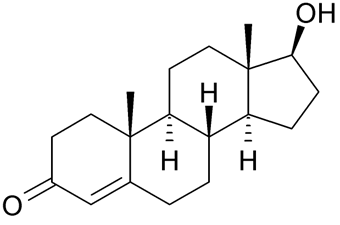 Testosterone
Testosterone Androsterone
Androsterone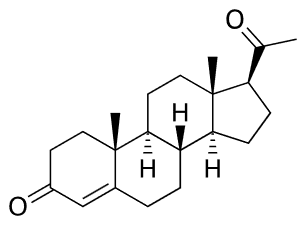 Estradiol
Estradiol
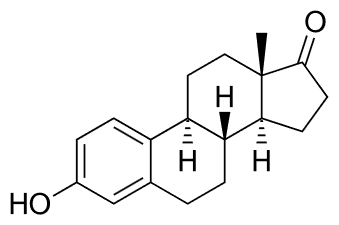 Estrone
Estrone
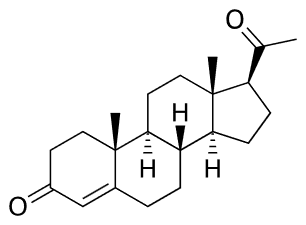 Progesterone
Progesterone
Functions of sex hormones
Testosterone and androsterone are the most potent male sex hormones. They control the development of male secondary sex characteristics, which include the growth of muscles, facial hair, maturation of sex organs, and sperms. Estrogens and progestins control the development of female secondary sex characteristics. Estrogens increase the size of the uterus, deposit fat in the breasts, and broaden the pelvis of females. Progestins prepare the uterus for nurturing the fertilized egg. If the egg is not fertilized, the levels of estrogens and progestins drop sharply, and menstruation begins.
The release of estrogens and progestins during pregnancy prevents ovulation. This is mimicked by synthetic estrogens, such as ethynyl estradiol, and synthetic progestins, such as norethindrone, shown below, used in birth control formulations. As with the use of any steroid hormones, there are risks associated with synthetic hormones for birth control, including weight gain and a greater chance of forming blood clots.
 Ethynyl estradiol
Ethynyl estradiol
 Norethindrone
Norethindrone
One physiological function of testosterone is increasing muscle mass and decreasing body fat. Several synthetic equivalents, called anabolic steroids, have been developed for use as appearance and performance-enhancing drugs, as shown in the figure below. Although there are some benefits of anabolic steroids, their harmful effects are numerous and outweigh their benefits. They may not produce a euphoric high but develop substance use disorder. They can cause early heart attacks, strokes, kidney failure, liver tumors, make pimples pop up, hair fallout, grow breasts, and reduced testicles in males, grow beards in females, and cause extreme mood changes that may lead to committing suicide. Some of these harmful effects are related to the changes in cholesterol levels, causing an increase in low-density lipoprotein (bad cholesterol), a decrease in high-density lipoprotein (good cholesterol), high blood pressure, and liver damage. The harmful effects are long-lasting and may be irreversible.
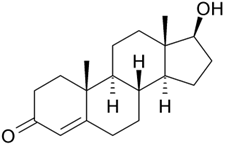 Testosterone
Testosterone Oxymetholone
Oxymetholone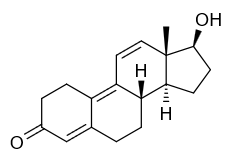 Trenbolone
Trenbolone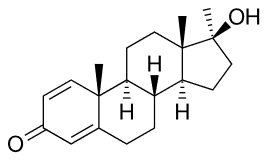 Methandrostenolone
Methandrostenolone Nandrolone
Nandrolone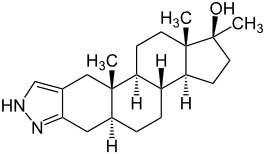 Stanozolol
Stanozolol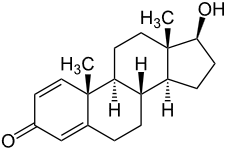 Boldenone
Boldenone Oxandrolone
OxandroloneAdrenocortical hormones
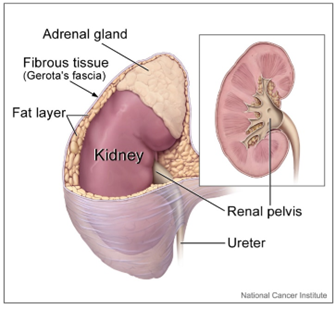 Adrenocortical hormones are produced by adrenal glands on the top of the kidneys, as shown in the figure on the right (Copyright; Alan Hoofring (Illustrator), Public domain, via Wikimedia Commons). There are two major subclasses: mineralocorticoids which regulate \(\ce{Na^{+}}\) and \(\ce{K^{+}}\) ions, and glucocorticoids which control carbohydrate metabolism.
Adrenocortical hormones are produced by adrenal glands on the top of the kidneys, as shown in the figure on the right (Copyright; Alan Hoofring (Illustrator), Public domain, via Wikimedia Commons). There are two major subclasses: mineralocorticoids which regulate \(\ce{Na^{+}}\) and \(\ce{K^{+}}\) ions, and glucocorticoids which control carbohydrate metabolism.
Mineralocorticoid
Aldosterone is an important mineralocorticoid that enhances the reabsorption of \(\ce{Na^{+}}\) and \(\ce{K^{+}}\) ions in the kidney tubules. The control of \(\ce{Na^{+}}\) and \(\ce{K^{+}}\) concentrations controls water absorption and swelling of tissues.
glucocorticoid
Cortisone is a glucocorticoid that increases blood glucose levels and glycogen synthesis in the liver. Cortisol is another glucocorticoid released under stress to increase blood glucose levels at the expense of the metabolism of carbohydrates, fats, and proteins.
These hormones or their synthetic derivative, such as prednisone, shown below, are used to reduce inflammation, asthma, and arthritis. As with the use of steroids in general, health problems can result if these steroids are used for the long term.
 Aldosterone
Aldosterone
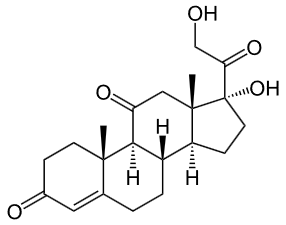 Cortisone
Cortisone
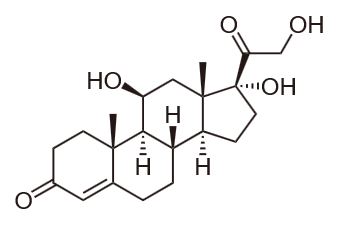 Cortisol
Cortisol
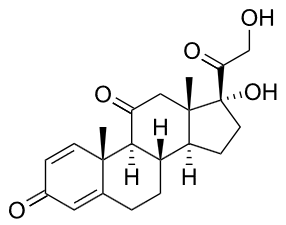 Prednisone (a synthetic corticoid)
Prednisone (a synthetic corticoid)


