1.4: Representing organic compounds
- Page ID
- 394087
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Read and draw molecular, Lewis, condensed, and structural formulae of simple organic compounds.
- Understand the mixed versions of and slight variations in drawing the formulae of simple organic compounds.
The molecular formula, Lewis formula, condensed formula, skeletal formula, or a combination of these can represent an organic compound. These ways of representing organic compound and what they mean is described below.
Molecular formula
The molecular formula tells the symbols of the elements that compose the compound, and the subscript to the element symbol denotes how many atoms of that element are in the molecule.
For example, (\(\ce{CH4}\)) is a molecule formula of methane which means there is one carbon and four hydrogen atoms in a methane molecule. \(\ce{C2H6}\) is a molecule formula of ethane, which means the ethane molecule has two carbon and six hydrogen atoms. Molecular formulas do not tell about the molecule's bonds and shapes.
Lewis formula
The Lewis structure or Lewis formula shows all the bonding electron pairs as lines (bonds) and lone pairs (non-bonding electron pairs) as pairs of dots around each atom in a molecule.
For example, Lewis formula of ethane (\(\ce{C2H6}\)) is: \(\ce{\small{H}-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}\!-H}\) that shows each carbon is bonded with one \(\ce{C}\) and three \(\ce{H}'s\) by single bonds. Similarly, Lewis formula of formaldehyde (\(\ce{CH2O}\)) is: \(\ce{\small{H}-{\underset{\underset{\Large{H}} |}{C}}=\overset{\Large{\cdot\cdot}}{O}\!:}\) that shows \(\ce{C}\) is bonded with two \(\ce{H}'s\) by single bonds and with one \(\ce{O}\) by a double bond and \(\ce{O}\) has two lone pairs on it. The lone pairs are usually omitted from Lewis structures except when needed to emphasize their presence. For example, Lewis formula for methanol (\(\ce{CH4O}\)) with lone pairs is: \(\ce{\small{H}-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}-\overset{\Large{\cdot\cdot}}{\underset{\Large{\cdot\cdot}}{O}}\!-H}\), but it can also be shown as \(\ce{\small{H}-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}-{O}\!-H}\) where the lone pairs are not shown on \(\ce{O}\) but it is understood that two lone pairs are there.
Condensed formula
The Lewis formulas become complicated and time-consuming for larger organic compounds.
Condensed formula simplifies the Lewis formula by writing each \(\ce{C}\) followed by \(\ce{H's}\) attached with it. Subscripts are used to show more than one \(\ce{H's}\). If there is a heteroatom, i.e., any atom other than \(\ce{C}\) or \(\ce{H}\) in the chain, it is condensed like \(\ce{C}\), except for halogens which are condensed like \(\ce{H}\).
For example, the Lewis formula of ethane \(\ce{\small{H}-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}\!-H}\) is condensed as \(\ce{CH3CH3}\). Lewis formula for methanol \(\ce{\small{H}-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}-{O}\!-H}\) is condensed as \(\ce{CH3OH}\). Lewis formula for ethanamine \(\ce{\small{H}-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}-{\underset{\underset{\Large{H}} |}{N}}\!-H}\) is condensed as \(\ce{CH3CH2NH2}\). Lewis formula for 2-chloropropane \(\ce{\small{H}-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}-\overset{\overset{\Large{Cl}}|}{\underset{\underset{\Large{H}} |}{C}}-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}\!-H}\), is condensed as \(\ce{CH3CHClCH3}\).
Skeletal formula
The Lewis and condensed formulas do not show the geometry of the organic compounds. Further, the Lewis and condensed formulas become complicated and time-consuming for large organic compounds.
Skeletal formulas or line-angle formulas overcome these drawbacks by simplifying the representation of organic compounds by omitting \(\ce{C's}\) and \(\ce{H's}\) in the formula and showing only the skeleton of the compound by \(\ce{C}\)-to-\(\ce{C}\) bonds as lines in a geometry that is closer to the actual geometry. Any heteroatom and \(\ce{H's}\) attached to the heteroatom are shown in the skeletal formula.
- Before learning skeletal formulas, it is essential to understand the following terms related to the primary type of organic compounds.
- Organic compounds containing only \(\ce{C's}\) and \(\ce{H's}\) are called hydrocarbons. For example, ethane (\(\ce{CH3CH3}\)), ethene (\(\ce{CH2CH2}\)), and ethyne (\(\ce{CHCH}\)) described in previous sections are hydrocarbons.
- The hydrocarbons contain only \(\sigma\)-bonds are called alkanes. For example, methane (\(\ce{CH4}\)) and ethane (\(\ce{CH3CH3}\)) are alkanes.
- Alkanes containing a chain of \(\ce{C's}\) where \(\ce{C's}\) are connected with either one or two \(\ce{C's}\) are called straight chain alkanes or normal-chain alkanes (n-alkanes). For example, n-alkane having four carbons is n-butane ((\(\ce{CH3CH2CH2CH3}\)), five carbons is n-pentane (\(\ce{CH3CH2CH2CH2CH3}\)), six carbons is n-hexane (\(\ce{CH3CH2CH2CH2CH2CH3}\)), and so on.
- Alkanes in which at least on \(\ce{C}\) connected with three or four other \(\ce{C's}\) are called branched-chain alkanes. For example, isopropane \(\ce{\small{H}-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}-\!\!\!\overset{\overset{\Large{H-\overset{\overset{\Large{H}}|}{C}\!-H}}|}{\underset{\underset{\Large{H}} |}{C}}\!\!\!-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}\!-H}\) is a branched-chain alkane
- The hydrocarbons containing at least one double bond, i.e., a \(\sigma\)-bond and a \(\pi\)-bond together, are called alkenes. For example, ethene
\(\ce{\small{H}-{\underset{\underset{\Large{H}} |}{C}}={\underset{\underset{\Large{H}} |}{C}}\!-H}\) is an alkene.
- The hydrocarbons containing at least one triple-bond, i.e., a \(\sigma\)-bond and two \(\pi\)-bonds together, are called alkynes. For example, ethyne \(\ce{\small{H-C≡C-H}}\) is an alkyne.
- The hydrocarbons with a planer cyclic structure having alternating odd numbers of double bonds are a special class of hydrocarbons called aromatic hydrocarbons that will be described later.
- Organic compounds containing at least one heteroatom, i.e., \(\ce{O}\), \(\ce{N}\), \(\ce{S}\), \(\ce{P}\), etc. are not hydrocarbons. For example, methanol \(\ce{CH3OH}\) is not a hydrocarbon. Several classes of organic compounds are not hydrocarbons, which will be described later.
Skeletal formulas of n-alkanes
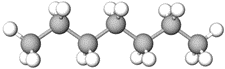
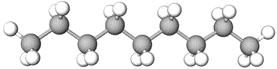
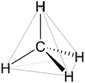
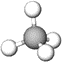
Methane (\(\ce{CH4}\)) is the simplest alkane that has a tetrahedral geometry around its \(\ce{C}\), as illustrated in Figure \(\PageIndex{1}\)a. Gray lines in Figure \(\PageIndex{1}\)a show the outline of the tetrahedron shape, and the black lines show \(\ce{C-H}\) bonds. The plane defined by \(\ce{C}\), \(\ce{H}\) on the top, and \(\ce{H}\) on the right is in the plane of the paper in this perspective drawing (Figure \(\PageIndex{1}\)a). The \(\ce{H}\) on the hashed wedge is going below, and the \(\ce{H}\) on the solid wedge is coming above the plane of the paper. The plane of the paper cuts through the middle of two \(\ce{H's}\) on the left of the drawing. The point of view is slightly above \(\ce{C}\) towards the top-right corner.
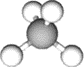
Figure \(\PageIndex{1}\)b shows the same structure without the tetrahedron layout drawing. All bonds are equal, and all bond angles are 109.5o. Figure \(\PageIndex{1}\)c shows the model of \(\ce{CH4}\) molecule from approximately the same view. Note that the geometry of \(\ce{CH4}\) molecule is two V's of 109.5o internal angle, placed perpendicular to each other, and joined at the vertex. Figure \(\PageIndex{1}\)d shows the same structure rotated such that the two \(\ce{H's}\) in the plane of the paper are on a straight line at the bottom of the drawing. Figure \(\PageIndex{1}\)e shows the model rotated in the same orientation as the perspective drawing in Figure \(\PageIndex{1}\)d.


Replacing any \(\ce{H}\) with another sp3 hybridized \(\ce{C}\) results in ethane (\(\ce{CH3CH3}\)) as shown in row two of Table 1. A line drawn to represent \(\ce{C-C}\) bond in ethane is the skeletal formula of ethane, as shown in row two of Table 1. When 2nd \(\ce{H}\) of methane is also replaced with another sp3 hybridized \(\ce{C}\), it results in propane (\(\ce{CH3CH2CH3}\)), as shown in row three of Table 1. Two \(\ce{H's}\) of methane in the plane of the page have been replaced with \(\ce{C's}\) in this case resulting in \(\ce{C-C-C}\) bonds in an inverted V-shape in the plane of the page. The skeletal formula representing a propane molecule is two lines connected in an inverted V-shape, as shown in row three of Table 1.
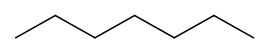
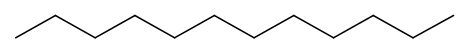
Replacing any \(\ce{H}\) of a terminal \(\ce{C}\) of a propane with another sp3 hybridized \(\ce{C}\) results in butane (\(\ce{CH3CH2CH2CH3}\)) as shown in 4th row of Table 1. Remember rotation around \(\ce{C-C}\) single bond happens. Therefore, the 4th \(\ce{C}\) of butane can be placed at any angle relative to the plane defined by the other three \(\ce{C's}\). However, placing all four \(\ce{C's}\) of butane in the same plane is the most stable arrangement because the terminal \(\ce{C's}\), which are the bulkiest groups attached to the internal \(\ce{C's}\), are farthest apart in this arrangement. Three lines connected by zigzag is the skeletal formula representing butane, as shown in row 4th of Table 1. The zigzag lines representing a chain of 4 \(\ce{C's}\) can be extended to represent a chain of 5 \(\ce{C's}\), by adding one line to represent a chain of 6 \(\ce{C's}\) by adding two lines, and so on, as shown in Table 1 for the cases of n-alkanes having a chain of 2 to 12 \(\ce{C's}\).
In summary, the skeletal formula of n-alkane is a line or lines connected zigzag representing \(\ce{C-C}\) bonds. It is understood that:
- the terminals (end) and corners (bends) of the lines are \(\ce{C's}\), and
- each \(\ce{C}\) has four bonds, so the bonds which are not shown by the lines are the bonds to \(\ce{H's}\).
With this knowledge, it is clear that a line or lines connected zigzag way are the structural formulas that represent structures of n-alkanes without showing \(\ce{C's}\) and \(\ce{H's}\) in the formula.
| # of \(\ce{C's}\) | Name | Molecular formula |
Model, Condensed formula, and Structural formula |
|---|---|---|---|
| 1 | Methane | \(\ce{CH4}\) |
\(\ce{CH4}\) |
| 2 | Ethane | \(\ce{C2H6}\) |
\(\ce{CH3CH3}\)
|
| 3 | Propane | \(\ce{C3H8}\) |
\(\ce{CH3CH2CH3}\)
|
| 4 | Butane | \(\ce{C4H10}\) |
\(\ce{CH3CH2CH2CH3}\)
|
| 5 | Pentane | \(\ce{C5H12}\) |
\(\ce{CH3CH2CH2CH2CH3}\)
|
| 6 | Hexane | \(\ce{C6H14}\) |
\(\ce{CH3CH2CH2CH2CH2CH3}\)
|
| 7 | Heptane | \(\ce{C7H16}\) |
\(\ce{CH3CH2CH2CH2CH2CH2CH3}\)
|
| 8 | Octane | \(\ce{C8H18}\) |
\(\ce{CH3CH2CH2CH2CH2CH2CH2CH3}\)
|
| 9 | Nonane | \(\ce{C9H20}\) |
\(\ce{CH3CH2CH2CH2CH2CH2CH2CH2CH3}\)
|
| 10 | Decane | \(\ce{C10H22}\) |
\(\ce{CH3CH2CH2CH2CH2CH2CH2CH2CH2CH3}\)
|
| 11 | Undecane | \(\ce{C11H24}\) |
\(\ce{CH3CH2CH2CH2CH2CH2CH2CH2CH2CH2CH3}\)
|
| 12 | Dodeane | \(\ce{C12H26}\) |
\(\ce{CH3CH2CH2CH2CH2CH2CH2CH2CH2CH2CH2CH3}\)
|
Names of n-alkanes without the last syllable, i.e., without -ane, are the stem names that represent the number of \(\ce{C's}\) in the organic compound. For example, meth- from methane represents one \(\ce{C}\), eth- from ethane represents two \(\ce{C's}\), prop- from propane represents three \(\ce{C's}\), and so on. These stem names will be described later in reference to name organic compounds.
It is evident from comparing the molecular formals of n-alkanes shown in Table 1 that n-alkanes differ from each other by a \(\ce{CH2}\) or a multiple of \(\ce{CH2}\) units. For example, add a \(\ce{CH2}\) to methane (\(\ce{CH4}\)) to convert it to propane (\(\ce{C2H6}\)), and two \(\ce{CH2}\) units to convert it to butane (\(\ce{C4H10}\) ) and so on. Series of organic compounds that differ from each other by a \(\ce{CH2}\) or multiple of \(\ce{CH2}\)'s are homologous series. All of the n-Alkanes shown in Table 1 are members of a homologous series.
The general formula of n-alkanes is \(\ce{C_{n}H_{2n + 2}}\) where n is a counting number, i.e., 1, 2, 3,... For example, when n = 1, it is methane \(\ce{C_{1}H_{2\times{1} + 2}}\) = \(\ce{CH4}\) (Recall: that when the subscript to the element symbol in the formal is 1, it is not written.). When n = 2, it is ethane \(\ce{C_{2}H_{2\times{2} + 2}}\) = \(\ce{C2H6}\), and so on
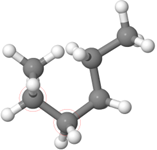
The structural formulas in Table 1 represent the linear conformation of n-alkanes which is the most stable confirmation. Remember: rotation is possible around any single bond. For example, n-hexane shown in Figure \(\PageIndex{2}\)a is rotated around the middle \(\ce{C-C}\) bond to acquire new confirmation shown in Figure \(\PageIndex{2}\)b and rotated further along the 2nd \(\ce{C-C}\) from left to acquire another confirmation shown in Figure \(\PageIndex{2}\)c. All three structural formulas are shown in Figure \(\PageIndex{2}\)a, b, and c represent the same molecule. Remember: different shapes of the same molecule obtained by rotation around a single bond are different configurations of the same molecule.
a)





Condensed and skeletal formulas of branched-alkanes
Replacing one or both \(\ce{H's}\) of a non-terminal carbon of straight chain alkane (n-alkane) with a \(\ce{C}\) or a \(\ce{C}\) chain results in a branched-alkane. Replacing hydrogen on the middle carbon of propane ( \(\ce{\small{H}-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}\!-H}\)) with a \(\ce{C}\) results in isopropane ( \(\ce{\small{H}-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}-\!\!\!\overset{\overset{\Large{H-\overset{\overset{\Large{H}}|}{C}\!-H}}|}{\underset{\underset{\Large{H}} |}{C}}\!\!\!-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}\!-H}\)) which is a branched chain alkane.
There are two ways to show the condensed formula of the branched alkane:
- show the condensed formula of the branch within small brackets next to the carbon it is attached to, e.g., \(\ce{CH3CH(CH3)CH3}\) is the condensed formula of isopropane;
- show the condensed formula of the branch hanging above or below the carbon it is attached, e.g., \(\ce{\small{CH3}-\overset{\overset{\LARGE{CH3\!\!\!\!\!\!\!}}|}{C}H-CH3}\) is the condensed formula of isopropane.
The skeletal formula of the branched alkane shows the skeletal formula of the branch with the terminal connected to the carbon in the main branch to which it is attached. For example,  is the skeletal formula of isopropane. Figure \(\PageIndex{3}\) shows another example of branched alkane and its condensed and skeletal formulas.
is the skeletal formula of isopropane. Figure \(\PageIndex{3}\) shows another example of branched alkane and its condensed and skeletal formulas.

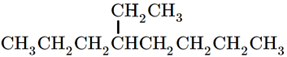 or \(\ce{CH3CH2CH2CH(CH2CH3)CH2CH2CH2CH3}\)
or \(\ce{CH3CH2CH2CH(CH2CH3)CH2CH2CH2CH3}\)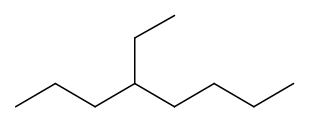
Skeletal formulas of alkenes
Hydrocarbons containing at least one double bond (\(\ce{C=C}\) bond) are called alkene.
Since the two sp2 \(\ce{C's}\) at the double bond have trigonal planer geometry with bond angles 120o, the double bond between the two sp2 \(\ce{C's}\) fit in the zigzag skeletal structure, like alkanes, except that two lines are drawn where there is a double bond in the chain.
Table 2 shows the names, Lewis structures, models, condensed formulas, and skeletal formulas of two alkene examples. Their nomenclature will be explained later.
| Name | Lewis structure |
Model, Condensed formula, and Structural formula |
|---|---|---|
| Hept-2-ene | \(\ce{\small{H}-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}-{\underset{\underset{\Large{H}} |}{C}}={\underset{\underset{\Large{H}} |}{C}}-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}\!-H}\) |
\(\ce{CH3CHCHCH2CH2CH2CH3}\)
|
| Propene | \(\ce{\small{H}-{\underset{\underset{\Large{H}} |}{C}}={\underset{\underset{\Large{H}} |}{C}}-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}\!-H}\) |
\(\ce{CH2CHCH3}\)
|
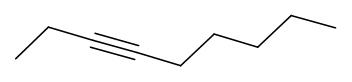
Skeletal formulas of alkynes
Hydrocarbons containing at least one triple bond (\(\ce{C≡C}\)) bond are called alkyne.
Since the two sp \(\ce{C's}\) at the triple-bond have linear geometry, three lines show the triple bond and the two single bonds to the other atoms attached to them are drawn in line with the triple bond. The zigzag skeletal structure shows the rest of the structure as usual.
Table 3 shows some alkyne examples' names, Lewis structures, models, condensed formulas, and skeletal formulas. Their nomenclature will be explained later.
| Name | Lewis structure |
Model, Condensed formula, and Structural formula |
|---|---|---|
| Propyne | \(\ce{\small{H}-{C}≡{C}-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}\!-H}\) |
\(\ce{CHCCH3}\)
|
| Pent-2-yne | \(\ce{\small{H}-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}-{C}≡{C}-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}\!-H}\) |
\(\ce{CH3CCCH2CCH3}\)
|
| Non-3-yne | \(\ce{\small{H}-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}-{C}≡{C}-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}\!-H}\) |
\(\ce{CH3CH2CCCH2CH2CH2CH2CH3}\)
|
Skeletal formulas of organic compounds containing heteroatoms
If any heteroatom, i.e., an atom other than \(\ce{C}\) or \(\ce{H}\), is present in an organic compound:
- its symbol in the skeletal formula shows it and
- any \(\ce{H}\) present on the heteroatom is also shown next to the heteroatom as in the condensed formulas.
The skeletal structure shows the rest of the structure as usual. Table 4 shows the names, Lewis structures, models, condensed formulas, and skeletal formulas of some example organic compounds containing heteroatoms. Their nomenclature will be explained later.
| Name | Lewis structure |
Model, Condensed formula, and Structural formula |
|---|---|---|
| 2-Chloropropane | \(\ce{\small{H}-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}-\overset{\overset{\Large{Cl}}|}{\underset{\underset{\Large{H}} |}{C}}-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}\!-H}\) |
\(\ce{CH3CHClCH3}\)
|
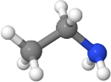
| Ethanamine | \(\ce{\small{H}-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}-{\underset{\underset{\Large{H}} |}{N}}\!-H}\) |
\(\ce{CH3CH2NH2}\)
|
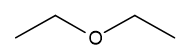
| Diethyl ether | \(\ce{\small{H}-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}-{O}-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}\!-H}\) |
\(\ce{CH3CH2OCH2CH3}\)
|
| Acetone | \(\ce{\small{H}-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}-\overset{\overset{\Large{O\!}}||}{C}-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}\!-H}\) |
\(\ce{CH3COCH3}\) or \(\ce{\small{CH3}\overset{\overset{\Large{O\!}}||}{C}{CH3}}\)
|
Variations in the formulas representing organic compounds
The condensed and the skeletal formulas are usually presented in organic chemistry, as described in the previous sections. However, they may be varied slightly according to the need.
If needed, mixed Lewis, condensed, and skeletal structures emphasize a particular part of the molecule.
For example, the \(\ce{H's}\) are usually written to the right of \(\ce{C}\) or to the right of heteroatoms in the condensed formula, but sometimes this order may be reversed. For example, Lewis formula for methanol \(\ce{\small{H}-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}-{O}\!-H}\) is presented in condensed form as \(\ce{CH3OH}\), but it may be condensed as \(\ce{H3COH}\), or as \(\ce{H3C-OH}\) to emphasize the \(\ce{C-O}\)-bond. Similarly, Lewis formula of acetone \(\ce{\small{H}-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}-\overset{\overset{\Large{O\!}}||}{C}-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}\!-H}\) is changed to skeletal formula as  , but it may also be shown as
, but it may also be shown as  to emphasize the \(\ce{C-H}\) bond.
to emphasize the \(\ce{C-H}\) bond.
Sometimes the formula is further condensed by placing the repeating units within a bracket and subscript outside the bract to show the number of repeat units. For example, the condensed formula of isopropane is \(\ce{\small{CH3}-\overset{\overset{\LARGE{CH3\!\!\!\!\!\!\!}}|}{C}H-CH3}\) that has three \(\ce{CH3}\) units attached to the central \(\ce{C}\). It can be further condensed as \(\ce{(CH3)3CH}\). Similarly, the condensed formula of decane \(\ce{CH3CH2CH2CH2CH2CH2CH2CH2CH2CH3}\) contains eight \(\ce{CH2}\) units and can be condensed further as \(\ce{CH3(CH2)8CH3}\).
Examples of drawing Lewis, condensed, and skeletal formulae
Draw molecular, Lewis, condensed, and skeletal formulas of butane.
Solution
But- in butane means four \(\ce{C's}\) and -ane means it is an alkane. Substituting n = 4 in the general formula of alkanes (\(\ce{C_{n}H_{n\times{2} + 2}}\)) gives:
molecular formula = (\(\ce{C_{4}H_{4\times{2} + 2} = C4H10}\).
For Lewis, condensed, and skeletal formulas follow the steps shown in the figure below.

Draw Lewis, condensed, and skeletal formulas for methylethylamine
Solution
Meth- means one \(\ce{C}\), eth-means two \(\ce{C's}\), and aminie indicates a \(\ce{N}\) two which the carbon chains are attached. With this information, follow the steps shown in the figure below.

Draw Lewis, condensed, and skeletal formula of 1-chlorobutane
Solution
But- means four \(\ce{C's}\) chain and 1-chloro- means there is chlorine at the terminal \(\ce{C}\). The nomenclature will be explained later. With this information, follow the steps shown in the figure below.