6.3: Strength of acids and bases
- Page ID
- 371800
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The strength of acid HA is the extent to which the acid dissociates into H+ and A- ions, as illustrated in Fig. 6.3.1.


Strong acids
Strong acids, like HCl, almost 100% dissociate into ions when they dissolve in water.
\begin{equation}
\mathrm{HCl}(\mathrm{g})+\mathrm{H}_{2} \mathrm{O}(\mathrm{l}) \rightarrow \mathrm{H}_{3} \mathrm{O}^{+}(\mathrm{aq})+\mathrm{Cl}^{-}(\mathrm{aq})\nonumber\end{equation}
One arrow is used to indicate that the reaction is nearly 100% complete.
Strong acids include HClO4, H2SO4, HI, HBr, HCl, and HNO3
Weak acids
Weak acids dissolve in water but partially dissociate into ions.
For example, acetic acid (CH3COOH) is a weak acid, 1 M acetic acid dissolves in water, but only 0.4% of the dissolved molecules dissociate into ions, the remaining 99.6% remain undissociated, as illustrated in Fig. 6.3.2. and equation of the dissociation equilibrium below.
\begin{equation}
\mathrm{CH}_{3} \mathrm{COOH}(\mathrm{aq})+\mathrm{H}_{2} \mathrm{O}(\mathrm{I}) \stackrel{\rightarrow}{\longleftarrow} \mathrm{H}_{3} \mathrm{O}^{+}(\mathrm{aq})+\mathrm{CH}_{3} \mathrm{COO}^{-}(\mathrm{aq})\nonumber
\end{equation}
Two arrows pointing in opposite directions are used for the dissociation of weak acids to indicate that the reaction is an equilibrium, i.e., two ways.
Often the arrows are not equal in size -the longer arrow points to acid-base pair that is weaker and present in a larger concentration at equilibrium than their conjugate pair.

Strong bases
Strong bases almost %100 dissociate into ions when dissolved in water. For example, NaOH is a strong base, and it dissociates almost 100% into ions in water.
Strong bases almost %100 dissociate into ions when dissolved in water. For example, NaOH is a strong base, and it dissociates almost 100% into ions in water.
\begin{equation}
\mathrm{NaOH}(\mathrm{s}) \stackrel{\text { Water }}{\longrightarrow} \mathrm{Na}^{+}(\mathrm{aq})+\mathrm{OH}^{-}(\mathrm{aq})\nonumber
\end{equation}
One arrow is used for the dissolution of strong bases to indicate that the reaction is almost complete.
Strong bases include hydroxides of alkali metals, i.e., LiOH, NaOH, KOH, RbOH, CsOH, and hydroxides of heavy alkaline earth metals, i.e., Ca(OH)2, Sr(OH)2, and Ba(OH)2.
The last three, i.e., the hydroxides of heavy alkaline earth metals, have low solubility in water, but the dissolved fraction exists as ions.
Weak bases
Weak bases partially dissociate into ions when dissolved in water.
For example, ammonia is a weak base –only 0.42% of the dissolved ammonia molecules dissociate into ammonium ions and hydroxide ions in water from a 1 M solution of ammonia.
\begin{equation}
\mathrm{NH}_{3}(\mathrm{aq})+\mathrm{H}_{2} \mathrm{O}(\mathrm{I}) \stackrel{\rightarrow}{\longleftarrow} \mathrm{NH}_{4}{ }^{+}(\mathrm{aq})+\mathrm{OH}^{-}(\mathrm{aq})\nonumber\
\end{equation}
Weak bases in household use include ammonia (NH3) in window cleaners, NaClO in bleach, Na2CO3 and Na3PO4 in laundry detergent, NaHCO3 in tooth past, Na2CO3 in baking powder, CaCO3 for use in lawns, Mg(OH)2 and Al(OH)3 in antacids and laxatives.
The weak bases mentioned above are all ionic compounds except ammonia. Ionic compounds are strong electrolytes, i.e., they dissociate into ions almost 100% upon dissolution in water. It appears to contradict the fact that these ionic compounds are weak bases. It does not actually contradict, because the base properties do not refer to these ionic compounds, the base properties refer to the reactions of their polyatomic anions, i.e., ClO-, CO32-, and PO43- with water, as shown in the reactions below:
\begin{equation}
\begin{aligned}
&\mathrm{ClO}^{-}+\mathrm{H}_{2} \mathrm{O} \stackrel{\rightarrow}{\longleftarrow} \mathrm{HClO}+\mathrm{OH}^{-} \\
&\mathrm{CO}_{3}^{2-}+2 \mathrm{H}_{2} \mathrm{O} \stackrel{\rightarrow}{\longleftarrow} \mathrm{H}_{2} \mathrm{CO}_{3}+2 \mathrm{OH}^{-} \text {, and } \\
&\mathrm{PO}_{4}{ }^{3-}+3 \mathrm{H}_{2} \mathrm{O} \stackrel{\rightarrow}{\longleftarrow} \mathrm{H}_{3} \mathrm{PO}_{4}+3 \mathrm{OH}^{-}
\end{aligned}\nonumber
\end{equation}
The above reactions are equilibrium reactions that are more favored in the revers than the forward direction, producing a small number of OH- ions compared to the anion on the reactant sides. The last two examples, i.e., Mg(OH)2 and Al(OH)2 are classified as weak bases because they are considered insoluble in water. The solubility of Mg(OH)2 is 0.00064 g/100 mL (25 °C), and the solubility of Al(OH)3 is 0.0001 g/100 mL, which are in the range of insoluble ionic compounds.
The solubility and the strength of acids and bases are two different things. A strong base may be less soluble, and a weak base may be more soluble or vice versa, but a dissolved strong base exists as ions only, and a dissolved weak base exists both as molecules and ions.
The relative strength of acid-conjugate base pair
A general rule is that the stronger the acid, the weaker the conjugate base, and vice versa.
The conjugate bases of strong acids have negligible base strength, and the conjugate acids of strong basses have negligible acid strength. Fig. 6.3.3. illustrates the relative strengths of some acids and their conjugated bases.
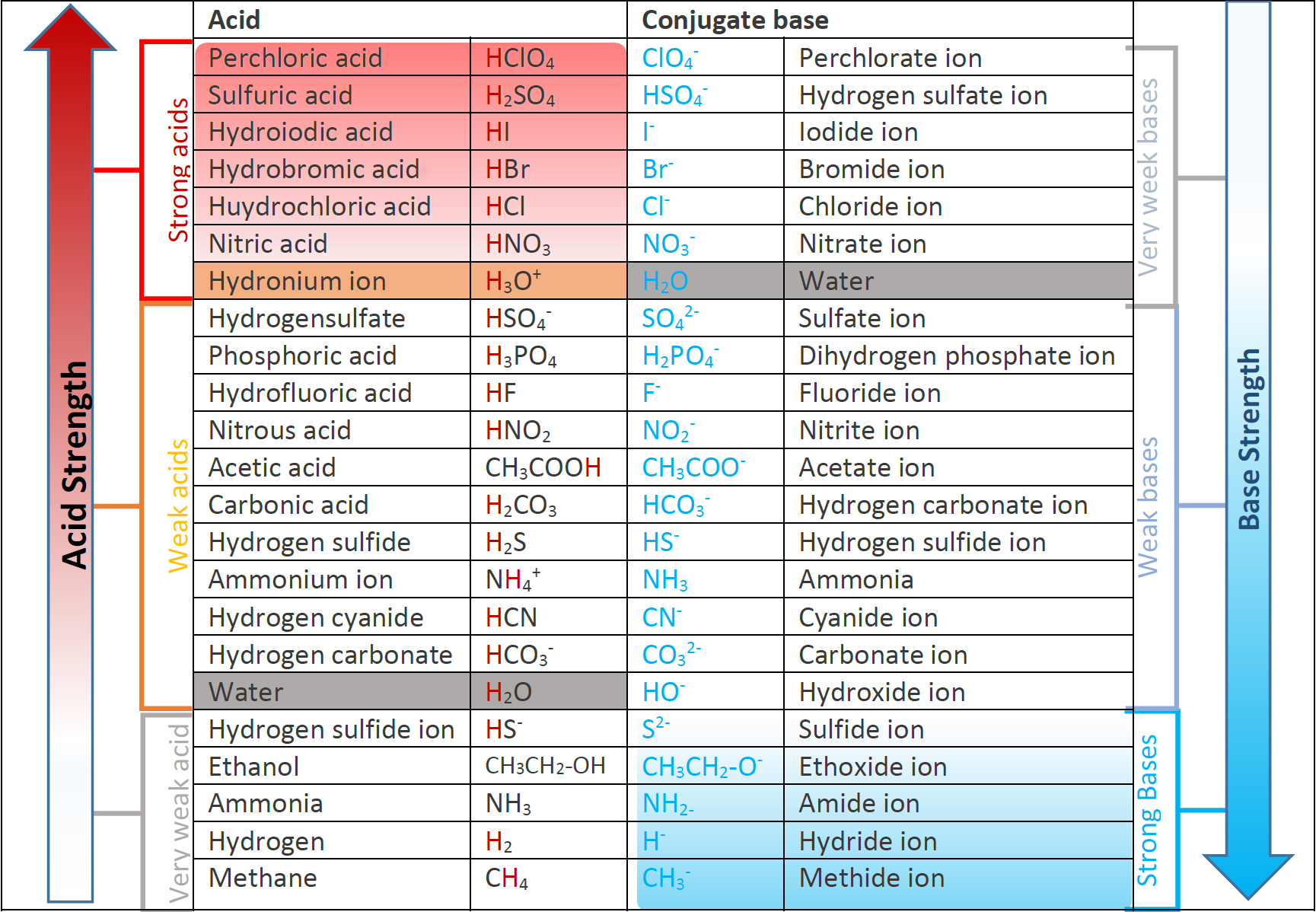
In any Brønsted–Lowry acid-base reaction, the general rule is that a stronger acid and a stronger base tend to form a weaker acid and a weaker base.
For example, a dissociation reaction between HCl and H2O is almost 100% complete because HCl is a stronger acid than H3O+ and H2O is a stronger base than Cl-:
\begin{equation}
\mathrm{HCl}+\mathrm{H}_{2} \mathrm{O} \rightarrow \mathrm{H}_{3} \mathrm{O}^{+}+\mathrm{Cl}^{-}\nonumber
\end{equation}
The dissolution of acetic acid (CH3COOH) and ammonia (NH3) are equilibrium reactions because all the acids, bases, and their conjugates are in the weak acids or weak bases category. However, acetic acid and water dominate over their conjugates H3O+ and CH3COO- by 99.6:0.4 ratio (in 1 M acetic acid solution) because the conjugate acid H3O+ is a stronger acid than CH3COOH, and conjugate base CH3COO- is a stronger base than H2O.
\begin{equation}
\mathrm{CH}_{3} \mathrm{COOH}(\mathrm{aq})+\mathrm{H}_{2} \mathrm{O}(\mathrm{l}) \stackrel{\rightarrow}{\longleftarrow} \mathrm{H}_{3} \mathrm{O}^{+}(\mathrm{aq})+\mathrm{CH}_{3} \mathrm{COO}^{-}(\mathrm{aq})\nonumber
\end{equation}
The longer arrow, in the unbalanced equilibrium arrows, points to the acid-base pair in the reaction that exists in a higher concentration relative to their conjugates.
Similarly, ammonia (NH3) and water (H2O) dominate over their conjugates NH4+ and OH- by ~99.6:0.4 ratio (1M ammonia solution) because the conjugate acid NH4+ is a stronger acid than H2O and conjugate base OH- is a stronger base than NH3.
\begin{equation}
\left.\mathrm{NH}_{3}(\mathrm{aq})+\mathrm{H}_{2} \mathrm{O}(\mathrm{I})\right) \stackrel{\rightarrow}{\longleftarrow} \mathrm{NH}_{4}^{+}(\mathrm{aq})+\mathrm{OH}^{-}(\mathrm{aq})\nonumber
\end{equation}


