4.3: Chemical reaction
- Page ID
- 372942
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)What is a chemical reaction?
A chemical reaction is a combination, separation, or rearrangement of atoms in a substance.
Atoms are healed together in a substance by chemical bonds. The combination makes bonds, separation breaks bonds, and rearrangement breaks some of the old bonds and makes some new bonds in the substances during a chemical reaction. It results in new substances with a different composition of elements than the starting substances. The starting substances are called reactants and the new substances formed are called products.
When a chemical bond is broken energy is required and when a chemical bond is formed energy is released. The amount of energy depended on the chemical bond, but for the same bond, the energy that needs to break is the same energy released to make the bond. Therefore, energy is released in some chemical reactions and absorbed in others.
Examples of chemical reactions are photosynthesis which converts carbon dioxide and water into glucose in the green leaves of plants using energy from sunlight. Digestion of food is a chemical reaction that releases the energy needed for the functioning of living things. The burning of a candle is a chemical reaction that converts the organic compounds in the fule to carbon dioxide, water, and heat energy. Rusting iron is another chemical reaction that converts the element iron to compound iron oxide.
Indications of a chemical reaction
A chemical reaction is usually accompanied by some physical changes that can be observed. The changes include color change, flame, heat, light, the evolution of a gas, formation of a precipitate, etc., as illustrated in Fig. 4.3.1




Representing a chemical reaction- a chemical equation
A chemical equation represents a chemical reaction.
- The formulas of the reactants are written on the left side separated by a + sign.
- Formulas of the products are written on the right-side separated by a + sign.
- An arrow pointing in the direction of products separates the reactants from the products.
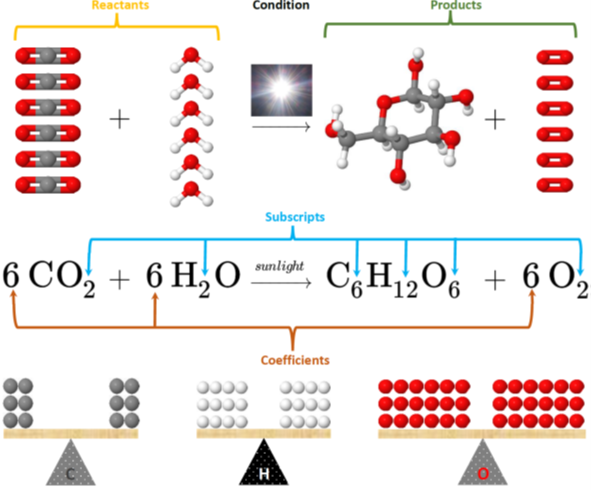
Fig. 4.3.2 illustrates the photosynthesis reaction between carbon dioxide (\(\ce{CO2}\) and water (\(\ce{H2O}\) producing glucose (\(\ce{C6H12O6}\) and oxygen (\(\ce{O2}\):
\[\ce{6CO2 + 6H2O ->[sun light] C6H12O6 + 6O2}\nonumber\]
- First correct formulas of reactants and products are written, separated by plus signs and arrows.
- Then the number of species is adjusted by adding a number, called a coefficient, at the beginning of a formula. The coefficients are needed to make atoms of each element equal on both sides of the equation because atoms are neither created nor destroyed in a chemical reaction.
- Subscripts within the formulas can not be changed as they represent the composition of the substance that is constant.
- The coefficient is not written if it is one.
For example, Fig. 4.3.2 shows coefficients 6 for carbon dioxide, water, and oxygen, but no coefficient for glucose means the coefficient is actually one for glucose in a balanced chemical equation for photosynthesis reaction.
Sometimes the physical state of the substances is shown by symbols in small brackets next to the formula.
- The symbols for the physical state are: (s) for solid, (l) for liquid, (g) for gas, and (aq) for a substance dissolved in water. The symbol (aq) stands for aqueous, i.e., water.
- If a gas evolves, it can be shown by an up-arrow (↑), and if a precipitate forms, it is shown by a down-arrow (↓).
For example, combustion of butane (\(ce{C4H10}\) in lighter shown in Fig. 4.3.1. can be represented as:
\[\ce{2C4H10(g) + 13O2(g) -> 8CO2(g) + 10H26O(g)}\nonumber\]
, where all the reactants and products are in the gas phase. Reaction of NaCl and AgNO3 in the water that results in a precipitate, as shown in Fig. 4.3.1 is:
\[\ce{NaCl(aq) + AgNO3(aq) -> AgCl(s) (v) + NaNO3(aq)}\nonumber\]
, where NaCl, AgNO3, and NaNO3 are in the water, and AgCl precipitates out as white solid. The reaction of limestone with HCl, shown in Fig. 4.3.1 is:
\[\ce{CaCO3(s) + 2HCl(aq) -> CaCl2(aq) + H2O(l) + CO2(g) ^}\nonumber\]
Reaction conditions, catalysts, or heat can be written above or below the arrow. Heat can also be represented by ∆ symbol, e.g.,
\[\ce{CaCO3(s) + CaO(s) ->[\Delta] CO2(g)^}\nonumber\]
A two way arrows \(\ce{<=>}\) or \(\leftrightarrows\) represents a two ways reaction, e.g.,
\[\ce{2NO2(g) <=> N2O4(g)}\nonumber\]
Balancing a chemical equation
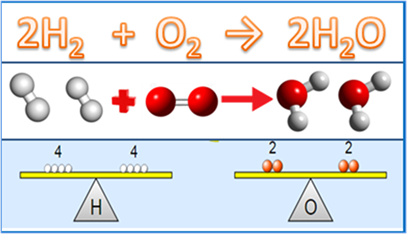
The first step is wright correct formulas of reactants and products separated by plus signs and an arrow. For example, the initial equation for burning hydrogen burns in oxygen and producing water would be:
\[\ce{H2 + O2 -> H2O}\nonumber\]
Note that hydrogen is written as H2 (not H) and oxygen as O2 (not O) because these reactants usually exist as molecules, not as atoms.
The next step is to add coefficients to balance atoms of each element on the two sides of the equation. For example, in the above equation hydrogen is balanced but oxygen is not. Balance oxygen by changing the coefficient of water from 1 to 2:
\[\ce{H2 + O2 -> 2H2O}\nonumber\]
Subscripts in the formulae can not be changed, as they are constant. For example, if O2 is changed to O in the above equation to balance the oxygen, it is incorrect as O2 is molecular oxygen which is a different chemical than atomic oxygen O.
The coefficient is a multiplier of each subscript in the formula, i.e., in 2H2O there are 2x2 = 4 hydrogen and 2x1 =2 oxygen. Now look for the other elements again: note that hydrogen atoms have changed to 4 on the right side. To balance hydrogen, change the coefficient of H2 from 1 to 2:
\[\ce{2H2 + O2 -> 2H2O}\nonumber\]
Check again: Now, the atoms of each element are the same on both sides, i.e., the equation is balanced, as illustrated in Fig. 4.3.3.
Rules to balance a chemical equation
The method described above to balance a chemical equation is called the hit and trial method. There are no hard and fast rules in this method. The general guidelines are:
- if an element occurs only in one reactant and one product, balance it first,
- if there is a free element in the reactants or products, balanced it last,
- if there is a polyatomic ion in reactants and products, balance the number of polyatomic ions as a unit, i.e., add coefficient to but do not change the subscripts in the polyatomic ion or of any formula.
- in some cases, a fractional coefficient is needed to balance the equation, in that case first use the fractional coefficient and when the equation is balanced, remove the fraction by multiplying all the coefficients with a common multiplier,
- if the set of coefficients in the balanced equation is not in the simplest whole-number ratio, the equation is still balanced, but it is recommended to convert the set of coefficients to the simplest whole-number ratio.
The following examples explain the guidelines.

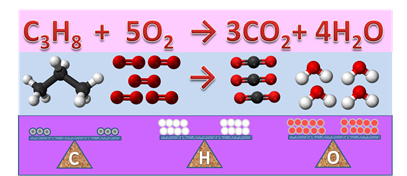
Combustion of propane (C3H8) is used in gas welding, as shown in Fig. 4.3.4. The propane reacts with oxygen and produces carbon dioxide and water. Write and balance the chemical equation of the reaction?
Solution
Start with writing the correct formulae of reactants and products in a chemical equation form:
\[\ce{C3H8 + O2 -> CO2 + H2O}\nonumber\]
According to rule 1, look at carbon or hydrogen first. Both are not balanced. Balance the carbon by changing the coefficient of CO2 from 1 to 3:
\[\ce{C3H8 + O2 -> 3CO2 + H2O}\nonumber\]
Balance the hydrogen by changing the coefficient of H2O from 1 to 4:
\[\ce{C3H8 + O2 -> 3CO2 + 4H2O}\nonumber\]
Now, look at oxygen (rule 2) and balance it by changing the coefficient of O2 from 1 to 5:
\[\ce{C3H8 + 5O2 -> 3CO2 + 4H2O}\nonumber\]
The last equation is balanced, as illustrated in Fig. 4.3.5.
The reaction between Aluminium metal powder and iron oxide, called thermite reaction, is highly exothermic that melts the ion. The thermite reaction is used for railroad welding as shown in Fig. 4.3.6. Wright and balance the reaction equation.
Solution
Start with writing the correct formulae of reactants and products in a chemical equation form:
\[\ce{Al + Fe2O3 -> Fe + Al2O3}\nonumber\]
Rule 1 does not apply as all elements are in one reactant and one product. According to rule 2, look at either one of the compounds. Balance aluminum by changing the coefficient in the eactant from 1 to 2:
\[\ce{2Al + Fe2O3 -> Fe + Al2O3}\nonumber\]
Now balance iron by change its coefficient from 1 to 2 in the product:
\[\ce{2Al + Fe2O3 -> 2Fe + Al2O3}\nonumber\]
All atoms are balanced at this stage and coefficients are in the simplest whole-number ratio. So the equation is balanced.

Methanol (CH4O) reacts with oxygen and produces carbon dioxide and water. Write and balance the chemical reaction equation?
Solution
The starting equation with correct formulas is:
\[\ce{CH4O + O2 -> CO2 + H2O}\nonumber\]
According to rule 1, look at carbon and hydrogen first. Carbon is already balanced. There are 4 hydrogens on the left but only two on the right side, so balance them by changing the coefficient of water from 1 to 2:
\[\ce{CH4O + O2 -> CO2 + 2H2O}\nonumber\]
Now, look at oxygen (rule 2). There are three oxygen atoms on the right (one in methanol and two in free element oxygen) but four oxygen on the right, it is not balanced. Changing the coefficient of CH4O from 1 to 2 balances the oxygen, but carbon and hydrogen go out of balance. Changing the coefficient of O2 from 1 to 2 does not balance the oxygen. A fractional coefficient 3/2 for O2 works at this stage (rule 4):
\[\ce{CH4O + 3/2 O2 -> CO2 + 2H2O}\nonumber\]
All elements are balanced, i.e., the equation is balanced, but, according to rule 5, it is better to remove the fraction by multiplying each coefficient in the equation with 2:
\[\ce{2CH4O + 3O2 -> 2CO2 + 4H2O}\nonumber\]
The set of coefficients in the balanced equation is already in the simplest whole-number ratio, so no further step is needed in this case.
Aluminum reacts sulfuric acid to produce aluminum sulfate and hydrogen gas. Wright a balanced chemical equation for the reaction.
Solution
The starting equation with correct formulas of reactants and products is:
\[\ce{Al + H2SO4 -> Al2(SO4)3 + H2}\nonumber\]
All elements occur in one reactant and one product, so rule 1 do not apply. According to rule 2, leave aluminum till the end and, according to rule 3, balance the polyatomic ion (SO42-) as a unit by changing the coefficient of sulfuric acid from 1 to 3:
\[\ce{Al + 3H2SO4 -> Al2(SO4)3 + H2}\nonumber\]
Now balance the hydrogen by changing it coefficient from 1 to 3:
\[\ce{Al + 3H2SO4 -> Al2(SO4)3 + 3H2}\nonumber\]
Finally balance aluminum by changing its coefficient from 1 to 2:
\[\ce{2Al + 3H2SO4 -> Al2(SO4)3 + 3H2}\nonumber\]
Double-check at the end –All elements are balanced, and all the coefficients are in the simplest whole-number ratio. No further step is needed.


