3.6: Lewis structures of molecules
- Page ID
- 372894
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Lewis symbols of elements
Lewis symbol of element shows the symbol of element with valence electrons shown as dots placed on top, bottom, left, and right sides of the symbol. Valence electrons up to four are shown as a single dot on either side of the symbol. The 5th, 6th, 7th, and 8th valence electron dots are paired with any of the first four dots. For example,  represent hydrogen, beryllium, boron, carbon, nitrogen, fluorine, and neon with 1, 2, 3, 4, 5, 6, 7, and 10 valence electrons, respectively. Lewis symbols of first twenty elements are shown in section 2.6, Table 1.
represent hydrogen, beryllium, boron, carbon, nitrogen, fluorine, and neon with 1, 2, 3, 4, 5, 6, 7, and 10 valence electrons, respectively. Lewis symbols of first twenty elements are shown in section 2.6, Table 1.
Lewis structures of simple molecules
An unpaired dot in the Lewis symbol of an element can make one bond by sharing it with an unpaired dot of another atom. The shared pair of dots represents a pair of bonding electrons, a covalent bond. For example, as shown below, a Hydrogen atom has one unpaired valence electron and makes a covalent bond with another hydrogen atom.
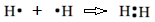
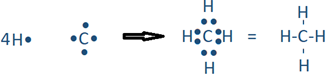
The covalent bond is usually represented by a single line between the bonded atoms, e.g., the H2 molecule shown in the above equation is generally shown as H-H. An example is a reaction between hydrogen having one valence electron and carbon having four valence electrons react to form CH4 molecule.
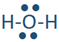
Similarly, hydrogen reacts with nitrogen, oxygen, and fluorine to form the following molecules: 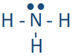 ,
,  , and
, and 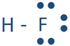 . Each line in these molecules represents a bonding electron pair, and the pair of dots represent valence electrons that are not involved in bonding, called lone pair of electrons. The lone pair is usually omitted from the Lewis structure unless it is needed to emphasize their presence for some reason.
. Each line in these molecules represents a bonding electron pair, and the pair of dots represent valence electrons that are not involved in bonding, called lone pair of electrons. The lone pair is usually omitted from the Lewis structure unless it is needed to emphasize their presence for some reason.
Procedure for writing Lewis structures of molecules
A systematic approach for writing the Lewis structure of molecules is explained with the help of the following example.
Draw the Lewis structures of CH4, PCl3, CO2, and HCN
Solution
Step 1: Add the valence electrons of all the molecules' atoms:
- CH4 has 4 valence electrons in C, and 1 in each of the four H: = 4 + 1x4 = 8 valence electrons
- PCl3 has 5 valence electros in P and 7 in each of the three Cl: = 5 + 7x3 = 26 valence electrons
- CO2 has 4 valence electrons in C and 6 in each of the two O: = 4 + 6x2 = 16 valence electrons
- HCN has 1 valence electron in H, 4 in C, and 5 in N: = 1 + 4 + 5 = 10 valence electrons
Step 2: Place the element symbol with more valances, i.e., having more unpaired dots in its Lewis structure, in the center and the rest of the atoms on four sides:
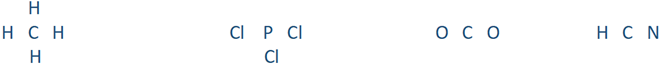
Step 3: Draw a line between the outer atom and the central atom to represent a single covalent bond:

Step 4: Every single bond consumes two valence electrons. Subtract the total number of valence electrons consumed in all the bonds from the total valence electrons initially present in step 1:
- CH4: 4 bond = 8 electrons consumed. 8 – 8 = 0 electrons left
- PCl3: 3 bond = 6 electrons consumed. 26 – 6 = 20 electrons left
- CO2: 2 bond = 4 electrons consumed. 16 – 4 = 12 electrons left
- HCN: 2 bond = 4 electrons consumed. 10 – 4 = 6 electrons left
Step 5: Distribute the remaining electrons as lone pairs, first to the outer atoms to complete their octet (duet in the case of hydrogen) and then to the central atom to complete its octet

Step 6: Check that the octet of each atom is complete (duet for hydrogen). If yes, the Lewis structure is complete, e.g., as in the cases of CH4 and PCl3 in the present examples. If not, move one of the lone pair of electrons from a neighboring atom to make a double bond, as shown by the red color arrows in the figure in the previous step. If the octet is still not complete, move one more lone pair of electrons from a neighboring atom: from the same atom to make a triple bond, as in the case of HCN above, or from another neighboring atom to make two double bonds, as in the case of CO2 above. The result is Lewis structures shown below.

To draw the Lewis structure of the most stable form, try to keep covalent bonds with an atom equal to the number of unpaired dots in the Lewis symbol of the atoms. For example, 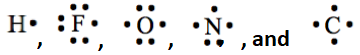 have 1, 1, 2, 3, and 4 unpaired electrons. So, hydrogen makes one, fluorine one, oxygen two, nitrogen 3, and carbon four covalent bonds in the stable molecules. When pulling a lone pair from neighboring atoms is needed to result in a double or triple bond, it is preferable to keep the resulting number of covalent bonds equal to the number of unpaired electrons in the Lewis symbol of the atom. For example, in the case of CO2 molecule, if both lone pairs were pulled from the same oxygen in step 6 above, the resulting Lewis structure would have been
have 1, 1, 2, 3, and 4 unpaired electrons. So, hydrogen makes one, fluorine one, oxygen two, nitrogen 3, and carbon four covalent bonds in the stable molecules. When pulling a lone pair from neighboring atoms is needed to result in a double or triple bond, it is preferable to keep the resulting number of covalent bonds equal to the number of unpaired electrons in the Lewis symbol of the atom. For example, in the case of CO2 molecule, if both lone pairs were pulled from the same oxygen in step 6 above, the resulting Lewis structure would have been 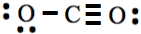 , which is technically correct Lewis structure but not the most stable form of this molecule. Note that the latter structure has one covalent bond in one oxygen and three covalent bonds in the other instead of two covalent bonds needed for oxygen.
, which is technically correct Lewis structure but not the most stable form of this molecule. Note that the latter structure has one covalent bond in one oxygen and three covalent bonds in the other instead of two covalent bonds needed for oxygen.
Exceptions to the octet rule
The octet rule generally applies in most cases, but there are exceptions in a few cases:
- Atoms of hydrogen, lithium, and beryllium tend to share, lose, or gain valence electrons to acquire the electron configuration of the nearest noble gas helium having two valence electrons. This is called the duet rule.
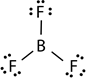
- Sometimes the atoms may end up in compounds with less than eight valence electrons. This often happens in the case of boron compounds and aluminum compounds of group 13. For example, boron has three valence electrons resulting in compounds like BF3 with three covalent bonds and six instead of eight valence electrons around boron.
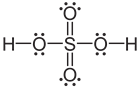
- Atoms of elements in period three and beyond sometimes end up in compounds with more than eight valence electrons. For example, PCl3 has octet complete, but PCl5 has 10 valence electrons. Similarly, sulfur has its octet complete in H2S but has 12 valence electrons in H2SO4. This happens because atoms in period thee and beyond have larger sizes and they have valence electrons in d or f orbitals in addition to the valence s and p orbitals. Table 1 lists some examples of exceptions to the octet rule.
| Structure | H:H | 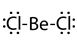 |
 |
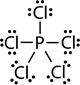 |
 |
|---|---|---|---|---|---|
| Name | Hydrogen | Beryllium dichloride | Boron trifluoride | Phosphorous pentafluoride | Sulfuric acid |
| Valence electrons on the central atom | 2 | 4 | 6 | 10 | 12 |


