3.9: Naming Ionic Compounds
- Page ID
- 86604
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Write the names for ionic compounds by recognizing and naming the ions in the formula unit.
Ionic compounds are named using the formula unit and by following some important conventions. First, the name of the cation is written first followed by the name of the anion. Because most metals form cations and most nonmetals form anions, formulas typically list the metal first and then the nonmetal. Second, charges are not included in the name (or the formula). Remember that in an ionic compound, the component species are ions, not neutral atoms, even though the formula does not contain charges. The proper formula for an ionic compound will show how many of each ion is needed to balance the total positive and negative charges; the name does not need to include indication of this ratio.
There are two main types of ionic compound with different naming rules for each; Type I: compounds containing cations of main group elements and Type II: compounds containing cations of variable charge (generally transition metals). Below we will look at examples of each type to learn the rules for naming.
Type I Ionic Compounds
Cations of main group elements do not have variable charges and are the simply named by placing the name of the cation first, followed by the name of the anion, and dropping the word ion from both parts. For example, what is the name of the compound whose formula is \(\ce{Ba(NO3)2}\)?
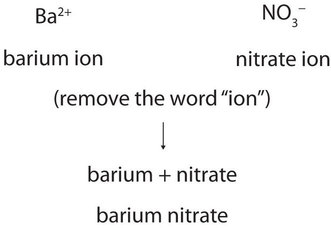
The compound’s name does not need to indicate that there are two nitrate ions for every barium ion. You must determine the ratio of ions in the formula unit by balancing the positive and negative charges.
Type II Ionic Compounds
Some metals can form cations with variable charges. When naming a formula for an ionic compound whose cation can have more than one possible charge, you must first determine the charge on the cation before identifying its correct name. For example, consider \(\ce{FeCl2}\) and \(\ce{FeCl3}\). In the first compound, the iron ion has a 2+ charge because there are two \(\ce{Cl^{−}}\) ions in the formula (1− charge on each chloride ion). In the second compound, the iron ion has a 3+ charge, as indicated by the three \(\ce{Cl^{−}}\) ions in the formula. These are two different compounds that need two different names. By the stock system, the names are iron(II) chloride and iron(III) chloride. If we were to use the stems and suffixes of the common system, the names would be ferrous chloride and ferric chloride, respectively.
Every day you encounter and use a large number of ionic compounds. Some of these compounds, where they are found, and what they are used for are listed in Table \(\PageIndex{1}\). Look at the label or ingredients list on the various products that you use during the next few days, and see if you run into any of those in this table, or find other ionic compounds that you could now name or write as a formula.
| Ionic Compound | Name | Use |
|---|---|---|
| NaCl | sodium chloride | ordinary table salt |
| KI | potassium iodide | added to “iodized” salt for thyroid health |
| NaF | sodium fluoride | ingredient in toothpaste |
| NaHCO3 | sodium bicarbonate | baking soda; used in cooking (and in antacids) |
| Na2CO3 | sodium carbonate | washing soda; used in cleaning agents |
| NaOCl | sodium hypochlorite | active ingredient in household bleach |
| CaCO3 | calcium carbonate | ingredient in antacids |
| Mg(OH)2 | magnesium hydroxide | ingredient in antacids |
| Al(OH)3 | aluminum hydroxide | ingredient in antacids |
| NaOH | sodium hydroxide | lye; used as drain cleaner |
| K3PO4 | potassium phosphate | food additive (many purposes) |
| MgSO4 | magnesium sulfate | added to purified water |
| Na2HPO4 | sodium hydrogen phosphate | anti-caking agent; used in powdered products |
| Na2SO3 | sodium sulfite | preservative |
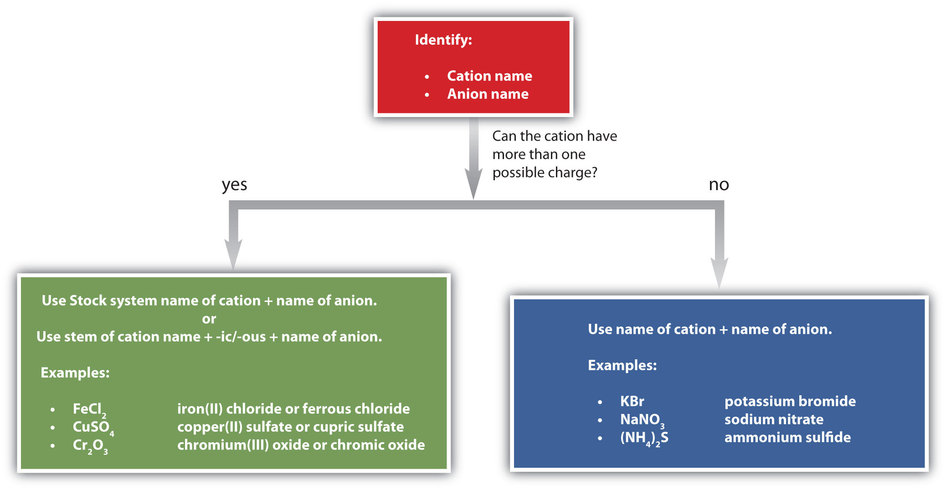
As you practice naming compounds, use Figure \(\PageIndex{1}\) as a guide.
Name each ionic compound, using both Stock and common systems if necessary.
- Ca3(PO4)2
- (NH4)2Cr2O7
- KCl
- CuCl
- SnF2
- Answer a
-
calcium phosphate
- Answer b
-
ammonium dichromate (the prefix di- is part of the name of the anion)
- Answer c
-
potassium chloride
- Answer d
-
copper(I) chloride or cuprous chloride
- Answer e
-
tin(II) fluoride or stannous fluoride
Name each ionic compound, using both Stock and common systems if necessary.
- ZnBr2
- Fe(NO3)3
- Al2O3
- CuF2
- AgF
- Answer a
-
zinc bromide
- Answer b
-
iron (III) nitrate or ferric nitrate
- Answer c
-
aluminum oxide
- Answer d
-
copper (II) fluoride or cupric fluoride
- Answer e
-
silver fluoride


