12.3: Metals and Ores
- Page ID
- 152212
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Identify important metals and describe their extraction from their main ores.
- List different metals, their uses, and their alloys.
- Describe the environmental impact of metal production.
Most metals are found as types of rock in the Earth's crust. These ores contain sufficient minerals with important elements including metals that can be economically extracted from the rock. Metal ores are generally oxides, sulfides, silicates (Table \(\PageIndex{1}\)) or "native" metals (such as native copper) that are not commonly concentrated in the Earth's crust, or "noble" metals (not usually forming compounds) such as gold (Figure \(\PageIndex{1}\)). The ores must be processed to extract the metals of interest from the waste rock and from the ore minerals.



Alloys
An alloy is a mixture composed of two or more elements, at least one of which is a metal. You are probably familiar with some alloys of copper (such as brass and bronze) and iron (steel) . Alloys can be one of two general types. In one type, called a substitutional alloy, the various atoms simply replace each other in the crystal structure. In another type, called an interstitial alloy, the smaller atoms such as carbon fit in between the larger atoms in the crystal packing arrangement.
Steels are a very important class of alloys. The many types of steels are primarily composed of iron, with various amounts of the elements carbon, chromium, manganese, nickel, molybdenum, and boron. Steels are widely used in building construction because of their strength, hardness, and resistance to corrosion. Most large modern structures like skyscrapers and stadiums are supported by a steel skeleton (see figure below).

Copper,Brass, and Bronze
Copper is a chemical element with the symbol Cu (from Latin: cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish-orange color. Copper is used as a conductor of heat and electricity and as a building material.
Copper is one of the few metals that can occur in nature in a directly usable metallic form (native metals). This led to very early human use in several regions, from c. 8000 BC. Thousands of years later, it was the first metal to be smelted from sulfide ores, c. 5000 BC, the first metal to be cast into a shape in a mold, c. 4000 BC and the first metal to be purposefully alloyed with another metal, tin, to create bronze, c. 3500 BC.
Most commercial ores are sulfides, especially chalcopyrite (CuFeS2), bornite (Cu5FeS4) and, to a lesser extent, covellite (CuS) and chalcocite (Cu2S).
Cu2S, is converted into oxides:
- 2 Cu2S + 3 O2 → 2 Cu2O + 2 SO2
The cuprous oxide is then converted to copper upon heating:
- 2 Cu2O → 4 Cu + O2
Copper is a constituent of various metal alloys, such as sterling silver used in jewelry, cupronickel used to make marine hardware and coins, and constantan used in strain gauges and thermocouples for temperature measurement. Bronze, an alloy of copper and tin has been in use since ancient times. The Bronze Age saw the increased use of metals rather than stone for weapons, tools, and decorative objects. Brass, an alloy of copper and zinc, is widely used in musical instruments like the trumpet and trombone. Alloys are commonly used in manufactured items because the properties of these metal mixtures are often superior to a pure metal. Bronze is harder than copper and more easily cast. Brass is very malleable and its acoustic properties make it useful for musical instruments (Figure \(\PageIndex{1}\)).

Iron and Steel
The early application of iron to the manufacture of tools and weapons was possible because of the wide distribution of iron ores and the ease with which iron compounds in the ores could be reduced by carbon. Iron ore is reduced with coke in a blast furnace (Figure \(\PageIndex{1}\)). The blast furnace is loaded with iron ores, usually hematite Fe2O3 or magnetite Fe3O4, together with coke (coal that has been separately baked to remove volatile components). Air pre-heated to 900 °C is blown through the mixture, in sufficient amount to turn the carbon into carbon monoxide:
- 2 C + O2 → 2 CO
This reaction raises the temperature to about 2000 °C The carbon monoxide reduces the iron ore to metallic iron[112]
- Fe2O3 + 3 CO → 2 Fe + 3 CO2
Some iron in the high-temperature lower region of the furnace reacts directly with the coke:[112]
- 2 Fe2O3 + 3 C → 4 Fe + 3 CO2
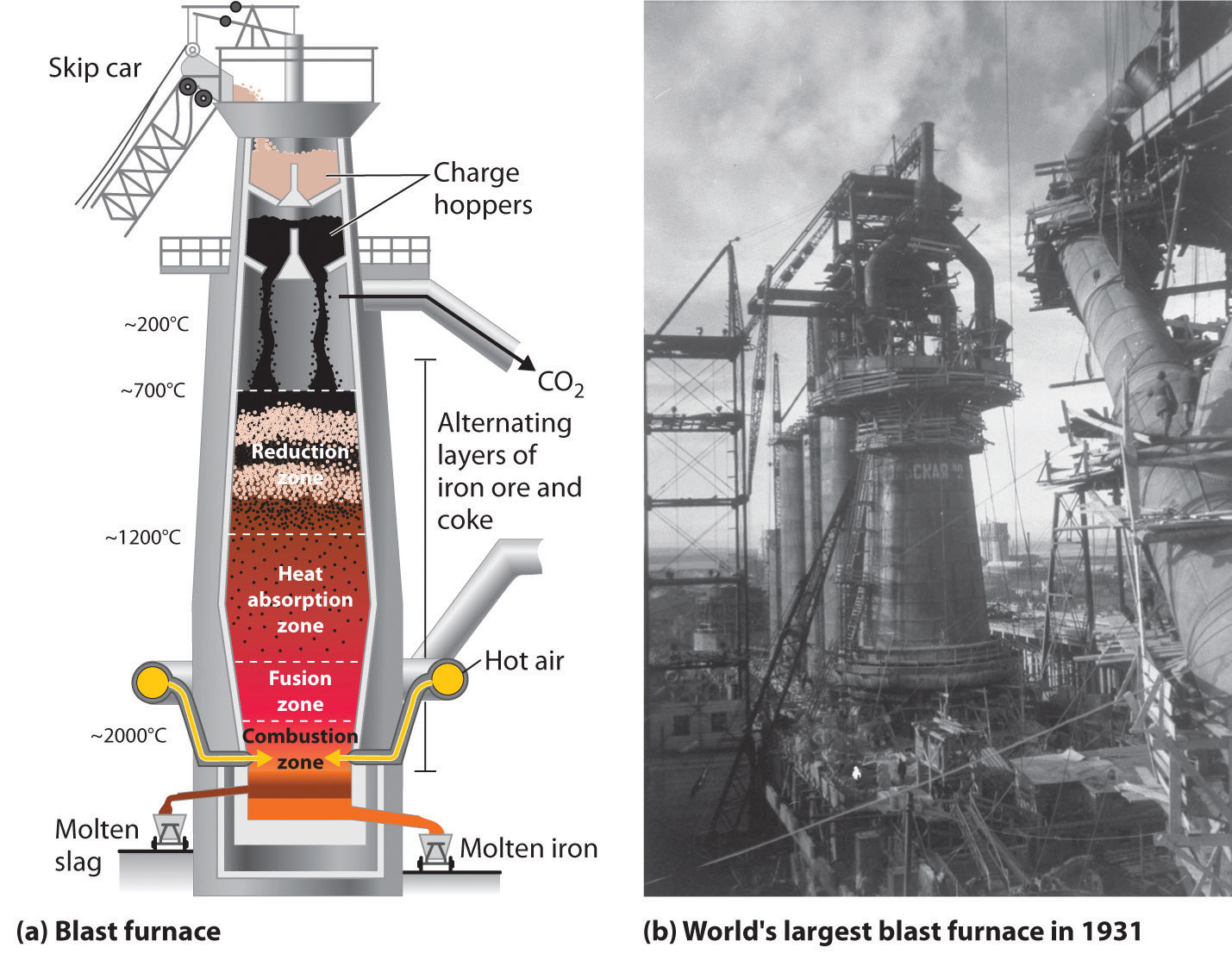
Much of the iron produced is refined and converted into steel. Steel is made from iron by removing impurities and adding substances such as manganese, chromium, nickel, tungsten, molybdenum, and vanadium to produce alloys with properties that make the material suitable for specific uses. Most steels also contain small but definite percentages of carbon (0.04%–2.5%). However, a large part of the carbon contained in iron must be removed in the manufacture of steel; otherwise, the excess carbon would make the iron brittle. However, there is not just one substance called steel - they are a family of alloys of iron with carbon or various metals.
Impurities in the iron from the Blast Furnace include carbon, sulfur, phosphorus and silicon, which have to be removed.
- Removal of sulfur: Sulfur has to be removed first in a separate process. Magnesium powder is blown through the molten iron and the sulfur reacts with it to form magnesium sulfide. This forms a slag on top of the iron and can be removed. \[ Mg + S \rightarrow MgS \label{127} \]
- Removal of carbon: The still impure molten iron is mixed with scrap iron (from recycling) and oxygen is blown on to the mixture. The oxygen reacts with the remaining impurities to form various oxides. The carbon forms carbon monoxide. Since this is a gas it removes itself from the iron! This carbon monoxide can be cleaned and used as a fuel gas.
- Removal of other elements: Elements like phosphorus and silicon react with the oxygen to form acidic oxides. These are removed using quicklime (calcium oxide) which is added to the furnace during the oxygen blow. They react to form compounds such as calcium silicate or calcium phosphate which form a slag on top of the iron.
Cast iron has already been mentioned above. This section deals with the types of iron and steel which are produced as a result of the steel-making process.
- Wrought iron: If all the carbon is removed from the iron to give high purity iron, it is known as wrought iron. Wrought iron is quite soft and easily worked and has little structural strength. It was once used to make decorative gates and railings, but these days mild steel is normally used instead.
- Mild steel: Mild steel is iron containing up to about 0.25% of carbon. The presence of the carbon makes the steel stronger and harder than pure iron. The higher the percentage of carbon, the harder the steel becomes. Mild steel is used for lots of things - nails, wire, car bodies, ship building, girders and bridges amongst others.
- High carbon steel: High carbon steel contains up to about 1.5% of carbon. The presence of the extra carbon makes it very hard, but it also makes it more brittle. High carbon steel is used for cutting tools and masonry nails (nails designed to be driven into concrete blocks or brickwork without bending). High carbon steel tends to fracture rather than bend if mistreated.
- Special steels: These are iron alloyed with other metals (Table \(\PageIndex{1}\)).
| Iron mixed with | Special properties | Uses include | |
|---|---|---|---|
| stainless steel | chromium and nickel | resists corrosion | cutlery, cooking utensils, kitchen sinks, industrial equipment for food and drink processing |
| titanium steel | titanium | withstands high temperatures | gas turbines, spacecraft |
| manganese steel | manganese | very hard | rock-breaking machinery, some railway track (e.g. points), military helmets |
Video \(\PageIndex{1}\) The process of steelmaking.
Aluminum
Aluminum is too high in the electrochemical series (reactivity series) to extract it from its ore using carbon reduction. The temperatures needed are too high to be economic. Instead, it is extracted by electrolysis. The ore is first converted into pure aluminum oxide by the Bayer Process, and this is then electrolyzed in solution in molten cryolite - another aluminum compound. The aluminum oxide has too high a melting point to electrolyse on its own. The usual aluminum ore is bauxite. Bauxite is essentially an impure aluminum oxide. The major impurities include iron oxides, silicon dioxide and titanium dioxide.
Crushed bauxite is treated with moderately concentrated sodium hydroxide solution. The concentration, temperature and pressure used depend on the source of the bauxite and exactly what form of aluminum oxide it contains. Temperatures are typically from 140°C to 240°C; pressures can be up to about 35 atmospheres. With hot concentrated sodium hydroxide solution, aluminum oxide reacts to give a solution of sodium tetrahydroxoaluminate.
\[ Al_2O_3 + 2NaOH + 3H_2O \longrightarrow 2NaAl(OH)_4 \nonumber \]
The sodium tetrahydroxoaluminate solution is cooled, and "seeded" with some previously produced aluminum hydroxide. This provides something for the new aluminum hydroxide to precipitate around.
\[ NaAl(OH)_4 \longrightarrow Al(OH)_3 + NaOH \nonumber \]
Aluminum oxide (sometimes known as alumina) is made by heating the aluminum hydroxide to a temperature of about 1100 - 1200°C.
\[ 2Al(OH)_3 \longrightarrow Al_2O_3 + 3H_2O \nonumber \]
The aluminum oxide is electrolyzed in solution in molten cryolite, Na3AlF6. Cryolite is another aluminum ore, but is rare and expensive, and most is now made chemically.
Uses of Aluminum
Aluminum is usually alloyed with other elements such as silicon, copper or magnesium. Pure aluminum isn't very strong, and alloying it adds to it strength. Aluminum is especially useful because it
- has a low density;
- is strong when alloyed;
- is a good conductor of electricity;
- has a good appearance;
- resists corrosion because of the strong thin layer of aluminum oxide on its surface. This layer can be strengthened further by anodizing the aluminum.
Anodizing essentially involves etching the aluminum with sodium hydroxide solution to remove the existing oxide layer, and then making the aluminum article the anode in an electrolysis of dilute sulfuric acid. The oxygen given of at the anode reacts with the aluminum surface, to build up a film of oxide up to about 0.02 mm thick. As well as increasing the corrosion resistance of the aluminum, this film is porous at this stage and will also take up dyes. (It is further treated to make it completely non-porous afterwards.) That means that you can make aluminum articles with the colour built into the surface.
Some uses include:
| Aluminum is used for | because |
|---|---|
| aircraft | light, strong, resists corrosion |
| other transport such as ships' superstructures, container vehicle bodies, tube trains (metro trains) | light, strong, resists corrosion |
| overhead power cables (with a steel core to strengthen them) | light, resists corrosion, good conductor of electricity |
| saucepans | light, resists corrosion, good appearance, good conductor of heat |
Recycling
Aluminum is an infinitely recyclable material, and it takes up to 95 percent less energy to recycle it than to produce primary aluminum, which also limits emissions, including greenhouse gases. Today, about 75 percent of all aluminum produced in history, nearly a billion tons, is still in use.[6]
The recycling of aluminum generally produces significant cost savings over the production of new aluminum, even when the cost of collection, separation and recycling are taken into account.[7] Over the long term, even larger national savings are made when the reduction in the capital costs associated with landfills, mines, and international shipping of raw aluminum are considered.
Environmental Impact of Steel and Aluminum Production
The impact of steel and aluminum production on the environment can be traced back from the mining of the ores to the production of the final commercial products (i.e. steel and aluminum). The main sources of emissions during the different phases of manufacture include the products of combustion such as nitrous oxide, carbon dioxide, carbon monoxide, and sulfur dioxide and fugitive dust from the operation of equipment. The effect of the different emissions on air quality (i.e. smog formation, greenhouse effect, acid rain etc.) will be discussed in more detail in Chapter 13.
Sulfuric acid is created when water and oxygen interact with sulfur bearing minerals and chemicals in rocks. Many metals become mobile as water becomes more acidic and at high concentrations these metals become toxic to most life forms. There is also production of enormous amounts of wastewater contaminants, hazardous wastes, and solid wastes.
Summary
- Metal ores contain sufficient minerals with important elements including metals that can be economically extracted from the rock. The ores must be processed to extract the metals of interest from the waste rock and from the ore minerals.
- Alloys are mixtures of materials, at least one of which is a metal.
- Bronze alloys were widely used in weapons.
- Brass alloys have long been employed in musical instruments.
- Steel alloys are strong and durable.
- Aluminum alloys are widely used due to its durability, resistance to corrosion, and good conductivity.
Contributors and Attributions
- TextMap: General Chemistry: General Chemistry (Petrucci et al.)
- Wikipedia

