12.2: Silicates and the Shapes of Things
- Page ID
- 152211
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Describe different types of silicate based minerals.
The silicates are the largest, the most interesting and the most complicated class of minerals than any other minerals. Approximately 30% of all minerals are silicates and some geologists estimate that 90% of the Earth's crust is made up of silicates, SiO44- based material. Thus, oxygen and silicon are the two most abundant elements in the earth's crust.
The building block of all of these minerals is the silica tetrahedron, a combination of four oxygen atoms and one silicon atom. As we’ve seen, it’s called a tetrahedron because planes drawn through the oxygen atoms form a shape with 4 surfaces (Figure \(\PageIndex{1}\)). Since the silicon ion has a charge of 4 and each of the four oxygen ions has a charge of −2, the silica tetrahedron has a net charge of −4.
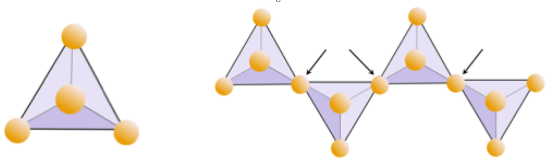
In silicate minerals, these tetrahedra are arranged and linked together in a variety of ways, from single units to complex frameworks (Table \(\PageIndex{1}\)).
| Tetrahedron Configuration Picture | Tetrahedron Configuration Name | Example Minerals |
|---|---|---|
 |
Isolated (nesosilicates) | Olivine, garnet, zircon, kyanite |
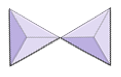 |
Pairs (sorosilicates) | Epidote, zoisite |
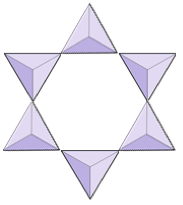 |
Rings (cyclosilicates) | Tourmaline |
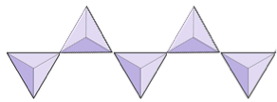 |
Single chains (inosilicates) | Pyroxenes, wollastonite |
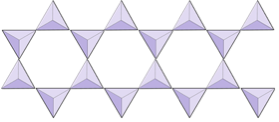 |
Double chains (inosilicates) | Amphiboles |
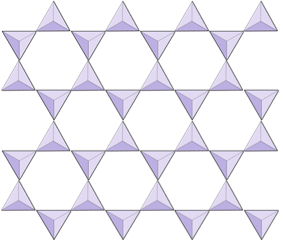 |
Sheets (phyllosilicates) | Micas, clay minerals, serpentine, chlorite |
| 3-dimensional structure | Framework (tectosilicates) | Feldspars, quartz, zeolite |
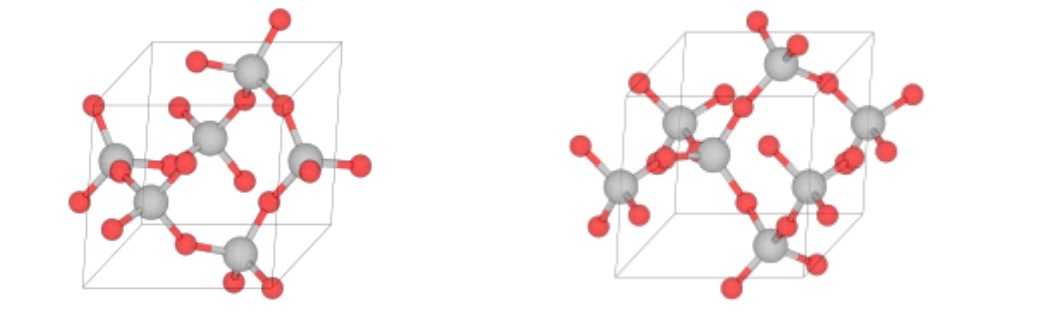
In the extreme case, the tetrahedra are arranged in a regular, orderly fashion forming a three-dimensional network. Quartz is such a structure (Figure \(\PageIndex{2}\)), and its formula is SiO2. If silica in the molten state is cooled very slowly it crystallizes at the freezing point. But if molten silica is cooled more rapidly, the resulting solid is a disorderly arrangement which is called glass, often also called quartz.
Silicates are extremely important materials, both natural (such as granite, gravel, and garnet) and artificial (such as Portland cement, ceramics, glass, and waterglass), for all sorts of technological and artistic activities. Source: Wikipedia
Glasses
Glass is a non-crystalline, often transparent amorphous solid, that has widespread practical, technological, and decorative use in, for example, window panes, tableware, optics, and optoelectronics. The most familiar, and historically the oldest, types of manufactured glass are "silicate glasses" based on the chemical compound silica (silicon dioxide, or quartz), the primary constituent of sand. The term glass, in popular usage, is often used to refer only to this type of material, which is familiar from use as window glass and glass bottles. Of the many silica-based glasses that exist, ordinary glazing and container glass is formed from a specific type called soda-lime glass, composed of approximately 75% silicon dioxide (SiO2), sodium oxide (Na2O) from sodium carbonate (Na2CO3), calcium oxide (CaO), also called lime, and several minor additives.
Table \(\PageIndex{2}\) list the more common types of silicate glasses and their ingredients, properties, and applications. Figure \(\PageIndex{3}\) are examples of silicate glasses.
| Type and Properties | Key Ingredients | Applications |
| Fused quartz has very low thermal expansion and excellent resistance to thermal shock, being able to survive immersion in water while red hot, resists high temperatures (1000–1500 °C) and chemical weathering, and is very hard. | Chemically pure silica (silicon dioxide) | Used for high-temperature applications such as furnace tubes, lighting tubes, melting crucibles, etc. |
| Soda-lime-silica glass is transparent, easily formed, and most suitable for window glass and tableware.[68] However, it has a high thermal expansion and poor resistance to heat. | (Na2O) + lime (CaO) + magnesia (MgO) + alumina (Al2O3) account for over 75% of manufactured glass, containing about 70 to 74% silica by weight | Typically used for windows, bottles, light bulbs, and jars.[ |
| Sodium borosilicate glasses have fairly low coefficients of thermal expansion (7740 Pyrex CTE is 3.25×10−6/°C[69] as compared to about 9×10−6/°C for a typical soda-lime glass[70]). They are, therefore, less subject to stress caused by thermal expansion and thus less vulnerable to cracking from thermal shock. | 5–13% boron trioxide (B2O3) | Commonly used for e.g. labware (e.g. Pyrex, Duran), household cookware, and sealed beam car head lamps.[ |
| Lead-oxide glass, crystal glass, lead glass has high density which results in a high electron density, and hence high refractive index, making the look of glassware more brilliant and causing noticeably more specular reflection and increased optical dispersion.[61][72] Lead glass has a high elasticity, making the glassware more workable and giving rise to a clear "ring" sound when struck. | silica + lead oxide (PbO) + potassium oxide (K2O) + soda (Na2O) + zinc oxide (ZnO) + alumina | Used for tableware, art objects, optical glass. |
| Aluminosilicate glass tends to be more difficult to melt and shape compared to borosilicate compositions, but has excellent thermal resistance and durability. | alumina and silica | Extensively used for fiberglass, used for making glass-reinforced plastics (boats, fishing rods, etc.), top-of-stove cookware, and halogen bulb glass. |
| Germanium-oxide glass is an extremely clear glass. Light loses only 5% of its intensity through 1 km of glass fiber. | alumina + germanium dioxide (GeO2). | Used for fiber-optic waveguides in communication networks. |
| Colored glass is clear glass with oxides added . | Different oxide additives produce the different colors in glass: turquoise (copper(II) oxide),[121] purple (manganese dioxide), red (cadmium sulfide), blue (cobalt oxide) and green (Iron(II) oxide and chromium(III) oxide). | Used for tableware and decorative glassware. |



a. b. c.
Figure \(\PageIndex{3}\) Examples of silicate glasses: a. Pyrex measuring cup, b. red glass bottle with yellow glass overlay, and c. four-color Roman glass bowl, manufactured circa 1st century B.C..
Asbestos
Asbestos is the name applied to six naturally occurring minerals that are mined from the earth. The different types of asbestos are:
- Amosite
- Chrysotile
- Tremolite
- ActinoliteUnlink
- Anthophyllite
- Crocidolite
Of these six, three are used more commonly. Chrysotile (white) is the most common, but it is not unusual to encounter. Amosite (brown / off-white), or Crocidolite (blue) as well. Asbestos are noncombustable fibrous material, and they have been used for terminal insulation material, brake linings, construction material, and filters. When mixed with cement, it reinforce the mechanical strength of concrete. It decomposes due to loss of water, and forms forsterite and silica at high temperature.
Ceramics
A ceramic is a solid material comprising an inorganic compound of metal, non-metal or ionic and covalent bonds. Common examples are earthenware, porcelain, and brick.
Varying crystallinity and electron composition in the ionic and covalent bonds cause most ceramic materials to be good thermal and electrical insulators (extensively researched in ceramic engineering). With such a large range of possible options for the composition/structure of a ceramic (e.g. nearly all of the elements, nearly all types of bonding, and all levels of crystallinity), the breadth of the subject is vast, and identifiable attributes (e.g. hardness, toughness, electrical conductivity, etc.) are difficult to specify for the group as a whole. General properties such as high melting temperature, high hardness, poor conductivity, high moduli of elasticity, chemical resistance and low ductility are the norm,[1] with known exceptions to each of these rules (e.g. piezoelectric ceramics, glass transition temperature, superconductive ceramics, etc.).
Cement and Concrete
A cement is a binder, a substance used for construction that sets, hardens, and adheres to other materials to bind them together. Cement is seldom used on its own, but rather to bind sand and gravel (aggregate) together. Cement mixed with fine aggregate produces mortar for masonry, or with sand and gravel, produces concrete. Cement is the most widely used material in existence and is only behind water as the planet's most-consumed resource.[1]
Cements used in construction are usually inorganic, often lime or calcium silicate based.
Concrete is a composite material composed of fine and coarse aggregate bonded together with a fluid cement (cement paste) that hardens over time—most frequently in the past a lime-based cement binder, such as lime putty, but sometimes with other hydraulic cements, such as a calcium aluminate cement or with Portland cement to form Portland cement concrete (for its visual resemblance to Portland stone).[2][3] Many other non-cementitious types of concrete exist with different methods of binding aggregate together, including asphalt concrete with a bitumen binder, which is frequently used for road surfaces, and polymer concretes that use polymers as a binder.
Summary
- The silicates are the largest, the most interesting and the most complicated class of minerals than any other minerals. Approximately 90% of the Earth's crust is made up of silicates, SiO44- based material.
- Silicates are extremely important materials, both natural (such as granite, gravel, and garnet) and artificial (such as Portland cement, ceramics, glass, and waterglass), for all sorts of technological and artistic activities
Contributors and Attributions
Chung (Peter) Chieh (Professor Emeritus, Chemistry @ University of Waterloo)
- Libretext: Physical Geology (Earle)
- Libretext: Inorganic Chemistry
- Wikipedia

