8.5: Oxygen - An Abundant and Essential Oxidizing Agent
- Page ID
- 152186
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\(\newcommand{\ket}[1]{\left| #1 \right>} \)
\( \newcommand{\bra}[1]{\left< #1 \right|} \)
\( \newcommand{\braket}[2]{\left< #1 \vphantom{#2} \right| \left. #2 \vphantom{#1} \right>} \)
\( \newcommand{\qmvec}[1]{\mathbf{\vec{#1}}} \)
\( \newcommand{\op}[1]{\hat{\mathbf{#1}}}\)
\( \newcommand{\expect}[1]{\langle #1 \rangle}\)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Know the occurrence and properties of oxygen.
- Know reactions involving oxygen as an oxidizing agent.
Elemental oxygen is a strong oxidizing agent. It reacts with most other elements and many compounds. Oxygen was isolated by Michael Sendivogius before 1604, but it is commonly believed that the element was discovered independently by Carl Wilhelm Scheele, in Uppsala, in 1773 or earlier, and Joseph Priestley in Wiltshire, in 1774. The name oxygen was coined in 1777 by Antoine Lavoisier, who first recognized oxygen as a chemical element and correctly characterized the role it plays in combustion.
Oxygen: Occurrence and Properties
By mass, oxygen is the third-most abundant element in the universe, after hydrogen and helium. Oxygen is the most abundant element on the earth’s crust. About 50% of the mass of the earth’s crust consists of oxygen (combined with other elements, principally silicon). Oxygen occurs as O2 molecules and, to a limited extent, as O3 (ozone) molecules in air. It forms about 20% of the mass of the air. About 89% of water by mass consists of combined oxygen. In combination with carbon, hydrogen, and nitrogen, oxygen is a large part of plants and animals.
Oxygen is a colorless, odorless, and tasteless gas at ordinary temperatures. It is slightly denser than air. Although it is only slightly soluble in water (49 mL of gas dissolves in 1 L at STP), oxygen’s solubility is very important to aquatic life.
Oxygen is essential in combustion processes such as the burning of fuels. All combustion reactions are also examples of redox reactions. A combustion reaction occurs when a substance reacts with oxygen to create heat. One example is the combustion of octane, the principle component of gasoline:
\[\ce{2 C_8H_{18} (l) + 25 O_2 (g) -> 16 CO_2 (g) + 18 H_2O (g) } \nonumber \]
Combustion reactions are a major source of energy for modern industry. Plants and animals use the oxygen from the air in respiration. The administration of oxygen-enriched air is an important medical practice when a patient is receiving an inadequate supply of oxygen because of shock, pneumonia, or some other illness.
The chemical industry employs oxygen for oxidizing many substances. A significant amount of oxygen produced commercially is important in the removal of carbon from iron during steel production. Large quantities of pure oxygen are also necessary in metal fabrication and in the cutting and welding of metals with oxyhydrogen and oxyacetylene torches. Liquid oxygen is important to the space industry. It is an oxidizing agent in rocket engines. It is also the source of gaseous oxygen for life support in space.
As we know, oxygen is very important to life. The energy required for the maintenance of normal body functions in human beings and in other organisms comes from the slow oxidation of chemical compounds. Oxygen is the final oxidizing agent in these reactions. In humans, oxygen passes from the lungs into the blood, where it combines with hemoglobin, producing oxyhemoglobin. In this form, blood transports the oxygen to tissues, where it is transferred to the tissues. The ultimate products are carbon dioxide and water. The blood carries the carbon dioxide through the veins to the lungs, where the blood releases the carbon dioxide and collects another supply of oxygen. Digestion and assimilation of food regenerate the materials consumed by oxidation in the body; the energy liberated is the same as if the food burned outside the body.
Oxygen: Reactions with Other Elements
Oxygen reacts directly at room temperature or at elevated temperatures with all other elements except the noble gases, the halogens, and few second- and third-row transition metals of low reactivity (those with higher reduction potentials than copper). Rust is an example of the reaction of oxygen with iron. The more active metals form peroxides or superoxides. Less active metals and the nonmetals give oxides. Two examples of these reactions are:
\[\ce{2Mg (s) + O2(g) -> 2MgO(s)} \nonumber \]
\[\ce{P4(s)+ 5O2 (g) -> P4O10(s)} \nonumber \]
The oxides of halogens, at least one of the noble gases, and metals with higher reduction potentials than copper do not form by the direct action of the elements with oxygen.
Oxygen: Reaction with Compounds
Elemental oxygen also reacts with some compounds. If it is possible to oxidize any of the elements in a given compound, further oxidation by oxygen can occur. For example, hydrogen sulfide, H2S, contains sulfur with an oxidation state of 2−. Because the sulfur does not exhibit its maximum oxidation state, we would expect H2S to react with oxygen. It does, yielding water and sulfur dioxide. The reaction is:
\[\ce{2H2S}(g)+\ce{3O2}(g)⟶\ce{2H2O}(l)+\ce{2SO2}(g) \nonumber \]
It is also possible to oxidize oxides such as CO and P4O6 that contain an element with a lower oxidation state. The ease with which elemental oxygen picks up electrons is mirrored by the difficulty of removing electrons from oxygen in most oxides. Of the elements, only the very reactive fluorine can oxidize oxides to form oxygen gas.
Most nonmetals react with oxygen to form nonmetal oxides. Depending on the available oxidation states for the element, a variety of oxides might form. Fluorine will combine with oxygen to form fluorides such as OF2, where the oxygen has a 2+-oxidation state.
Sulfur Oxygen Compounds
The two common oxides of sulfur are sulfur dioxide, SO2, and sulfur trioxide, SO3. The odor of burning sulfur comes from sulfur dioxide. Sulfur dioxide, occurs in volcanic gases Figure \(\PageIndex{1}\) and in the atmosphere near industrial plants that burn fuel containing sulfur compounds.

Ozone: Another Form of Oxygen
When dry oxygen is passed between two electrically charged plates, ozone (O3, illustrated in Figure \(\PageIndex{2}\)), an allotrope of oxygen possessing a distinctive odor, forms. The formation of ozone from oxygen is an endothermic reaction, in which the energy comes from an electrical discharge, heat, or ultraviolet light:
\[\ce{3O2}(g)\xrightarrow{\ce{electric\: discharge}}\ce{2O3}(g) \nonumber \]
The sharp odor associated with sparking electrical equipment is due, in part, to ozone.
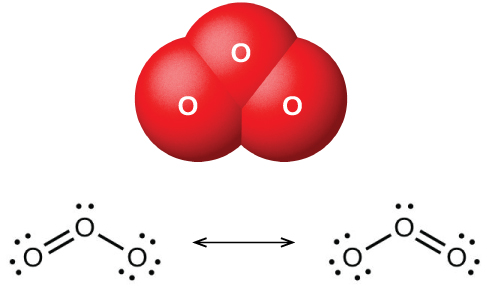
Ozone forms naturally in the upper atmosphere by the action of ultraviolet light from the sun on the oxygen there. Most atmospheric ozone occurs in the stratosphere, a layer of the atmosphere extending from about 10 to 50 kilometers above the earth’s surface. This ozone acts as a barrier to harmful ultraviolet light from the sun by absorbing it via a chemical decomposition reaction:
\[\ce{O3}(g)\xrightarrow{\ce{ultraviolet\: light}}\ce{O}(g)+\ce{O2}(g) \nonumber \]
The reactive oxygen atoms recombine with molecular oxygen to complete the ozone cycle. The presence of stratospheric ozone decreases the frequency of skin cancer and other damaging effects of ultraviolet radiation. It has been clearly demonstrated that chlorofluorocarbons, CFCs (known commercially as Freons), which were present as aerosol propellants in spray cans and as refrigerants, caused depletion of ozone in the stratosphere. This occurred because ultraviolet light also causes CFCs to decompose, producing atomic chlorine. The chlorine atoms react with ozone molecules, resulting in a net removal of O3 molecules from stratosphere. This process is explored in detail in our coverage of chemical kinetics. There is a worldwide effort to reduce the amount of CFCs used commercially, and the ozone hole is already beginning to decrease in size as atmospheric concentrations of atomic chlorine decrease. While ozone in the stratosphere helps protect us, ozone in the troposphere is a problem. This ozone is a toxic component of photochemical smog.
The uses of ozone depend on its reactivity with other substances. It can be used as a bleaching agent for oils, waxes, fabrics, and starch: It oxidizes the colored compounds in these substances to colorless compounds. It is an alternative to chlorine as a disinfectant for water.
Low level ozone (or tropospheric ozone) is an atmospheric pollutant.[42] It is not emitted directly by car engines or by industrial operations, but formed by the reaction of sunlight on air containing hydrocarbons and nitrogen oxides that react to form ozone directly at the source of the pollution or many kilometers down wind.
Ozone reacts directly with some hydrocarbons such as aldehydes and thus begins their removal from the air, but the products are themselves key components of smog.
Ozone can be used to remove iron and manganese from water, forming a precipitate which can be filtered:
\[2 Fe^{2+} + O_3 + 5 H_2O → 2 Fe(OH)_3(s) + O_2 + 4 H^+ \nonumber \]
\[2 Mn^{2+} + 2 O_3 + 4 H_2O → 2 MnO(OH)_2(s) + 2 O_2 + 4 H^+ \nonumber \]
Ozone will also oxidize dissolved hydrogen sulfide in water to sulfurous acid:
\[3 O_3 + H_2S → H_2SO_3 + 3 O_2 \nonumber \]
These three reactions are central in the use of ozone based well water treatment.
Ozone will also detoxify cyanides by converting them to cyanates.
\[CN^− + O_3 → CNO^− + O_2 \nonumber \]
Ozone will also completely decompose urea:
\[(NH_2)_2CO + O_3 → N_2 + CO_2 + 2 H_2O \nonumber \]
Other Common Oxidizing Agents
Chlorine is usually used (in the form of hypochlorous acid) to kill bacteria and other microbes in drinking water supplies and public swimming pools. In most private swimming pools, chlorine itself is not used, but rather sodium hypochlorite, formed from chlorine and sodium hydroxide, or solid tablets of chlorinated isocyanurates. The drawback of using chlorine in swimming pools is that the chlorine reacts with the proteins in human hair and skin. The distinctive 'chlorine aroma' associated with swimming pools is not the result of chlorine itself, but of chloramine, a chemical compound produced by the reaction of free dissolved chlorine with amines in organic substances. As a disinfectant in water, chlorine is more than three times as effective against Escherichia coli as bromine, and more than six times as effective as iodine. Increasingly, chloramine itself is being directly added to drinking water for purposes of disinfection, a process known as chloramination.
It is often impractical to store and use poisonous chlorine gas for water treatment, so alternative methods of adding chlorine are used. These include hypochlorite solutions, which gradually release chlorine into the water, and compounds like sodium dichloro-s-triazinetrione (dihydrate or anhydrous), sometimes referred to as "dichlor", and trichloro-s-triazinetrione, sometimes referred to as "trichlor". These compounds are stable while solid and may be used in powdered, granular, or tablet form. When added in small amounts to pool water or industrial water systems, the chlorine atoms hydrolyze from the rest of the molecule forming hypochlorous acid (HOCl), which acts as a general biocide, killing germs, micro-organisms, algae, and so on.
Breathalizers
The breathalyzer is a redox reaction. When the potassium dichromate reacts with ethanol it loses an oxygen atom (gets reduced), going from the orange dichromate to the green chromium sulfate. At the same time dichromate is being reduced, ethanol gains an oxygen atom (gets oxidized), forming acetic acid. The sulfuric acid helps to remove the ethanol from the exhaled air into the test solution and also provides the necessary acidic conditions.
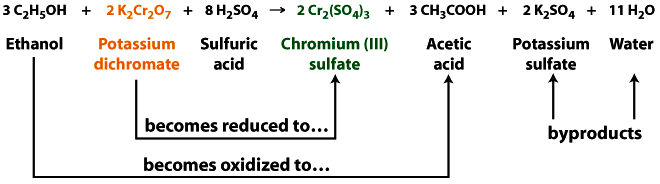
In this reaction, the chromium atom is reduced from \(Cr^{6+}\) to \(Cr^{3+}\), and the ethanol is oxidized to acetic acid.
Hydrogen peroxide can be used for the sterilization of various surfaces, including surgical tools and may be deployed as a vapor (VHP) for room sterilization. \(\ce{H2O2}\) demonstrates broad-spectrum efficacy against viruses, bacteria, yeasts, and bacterial spores. In general, greater activity is seen against Gram-positive than Gram-negative bacteria; however, the presence of catalase or other peroxidases in these organisms can increase tolerance in the presence of lower concentrations. Higher concentrations of \(\ce{H2O2}\) (10 to 30%) and longer contact times are required for sporicidal activity.
Hydrogen peroxide is seen as an environmentally safe alternative to chlorine-based bleaches, as it degrades to form oxygen and water and it is generally recognized as safe as an antimicrobial agent by the U.S. Food and Drug Administration (FDA).
Historically hydrogen peroxide was used for disinfecting wounds, partly because of its low cost and prompt availability compared to other antiseptics. It is now thought to inhibit healing and to induce scarring because it destroys newly formed skin cells. Only a very low concentration of
\(\ce{H2O2}\) can induce healing, and only if not repeatedly applied. Surgical use can lead to gas embolism formation. Despite this, it is still used for wound treatment in many countries but is also prevalent as a major first aid antiseptic in the United States
Diluted \(\ce{H2O2}\) (between 1.9% and 12%) mixed with aqueous ammonia is used to bleach human hair. The chemical's bleaching property lends its name to the phrase "peroxide blonde". Hydrogen peroxide is also used for tooth whitening. It can be found in most whitening toothpastes.
Almost all applications of potassium permanganate (\(\ce{KMnO4}\)) exploit its oxidizing properties. As a strong oxidant that does not generate toxic byproducts, \(\ce{KMnO4}\) has many niche uses. Potassium permanganate is used for a number of skin conditions. This includes fungal infections of the foot, impetigo, pemphigus, superficial wounds, dermatitis, and tropical ulcers. It is on the WHO Model List of Essential Medicines, the most important medications needed in a basic health system. Potassium permanganate is used extensively in the water treatment industry.
Benzoyl peroxide (BPO) is a medication and industrial chemical. As a medication, it is used to treat mild to moderate acne. For more severe cases, it may be used with other treatments. Some versions are sold mixed with antibiotics such as clindamycin. Other uses include bleaching flour, hair bleaching, teeth whitening, and textile bleaching.[5] It is also used in the plastic industry
Sodium hypochlorite is most often encountered as a pale greenish-yellow dilute solution commonly known as liquid bleach or simply bleach, a household chemical widely used (since the 18th century) as a disinfectant or a bleaching agent. The compound in solution is unstable and easily decomposes, liberating chlorine, which is the active principle of such products. Indeed, sodium hypochlorite is the oldest and still most important chlorine-based bleach.
While sodium hypochlorite is non-toxic, its corrosive properties, common availability, and reaction products make it a significant safety risk. In particular, mixing liquid bleach with other cleaning products, such as acids or ammonia, may produce toxic fumes.
Summary
- Oxygen is one of the most reactive elements. This reactivity, coupled with its abundance, makes the chemistry of oxygen very rich and well understood.
- Many compounds of the representative metals and non metals react with oxygen to form oxides.
- Other than oxygen, ozone, chlorine, potassium dichromate, hydrogen peroxide, potassium peroxide, benzoyl peroxide, sodium hypochlorite are common oxidizing agents mentioned in this section, that have many beneficial uses.
Contributors and Attributions
Paul Flowers (University of North Carolina - Pembroke), Klaus Theopold (University of Delaware) and Richard Langley (Stephen F. Austin State University) with contributing authors. Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. Download for free at http://cnx.org/contents/85abf193-2bd...a7ac8df6@9.110).
- Wikipedia

