8.2: Oxidizing and Reducing Agents
- Page ID
- 152183
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Identify oxidizing and reducing agents.
Life on planet Earth is a complicated and well-organized set of processes. Animals are designed to breathe oxygen and plants are designed to produce oxygen. Photosynthesis is the means by which we get the oxygen we need for life. Light striking a plant pigment known as chlorophyll initiates a complex series of reactions, many of which involve redox processes complete with movement of electrons. In this series of reactions, water is converted to oxygen gas, and we have something to sustain our lives.
The reaction below is a redox reaction that produces zinc sulfide:
\[\ce{Zn} + \ce{S} \rightarrow \ce{ZnS}\nonumber \]
The half-reactions can be written:
\[\begin{align*} &\text{Oxidation:} \: \ce{Zn} \rightarrow \ce{Zn^{2+}} + 2 \ce{e^-} \\ &\text{Reduction:} \: \ce{S} + 2 \ce{e^-} \rightarrow \ce{S^{2-}} \end{align*} \nonumber \]
In the reaction above, zinc is being oxidized by losing electrons. However, there must be another substance present that gains those electrons and in this case that is the sulfur. In other words, the sulfur is causing the zinc to be oxidized. Sulfur is called the oxidizing agent. The zinc causes the sulfur to gain electrons and become reduced and so the zinc is called the reducing agent. The oxidizing agent is a substance that causes oxidation by accepting electrons; therefore, its oxidation state decreases. The reducing agent is a substance that causes reduction by losing electrons; therefore its oxidation state increases. The simplest way to think of this is that the oxidizing agent is the substance that is reduced, while the reducing agent is the substance that is oxidized as shown in Figure \(\PageIndex{1}\) and summarized in Table \(\PageIndex{1}\).
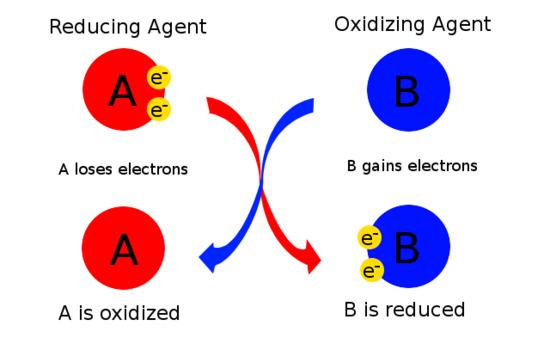
Both the oxidizing and reducing agents are the reactants and therefore appear on the left-hand side of an equation.
| Oxidizing Agents | Reducing Agents | |
|---|---|---|
| Oxidation State | Decreases | Increases |
| # of Electrons | Gained | Lost |
| Substance is... | Reduced | Oxidized |
The examples below show how to analyze a redox reaction and identify oxidizing and reducing agents.
When chlorine gas is bubbled into a solution of sodium bromide, a reaction occurs which produces aqueous sodium chloride and bromine. Determine what is being oxidized and what is being reduced. Identify the oxidizing and reducing agents.
\[\ce{Cl_2} \left( g \right) + 2 \ce{NaBr} \left( aq \right) \rightarrow 2 \ce{NaCl} \left( aq \right) + \ce{Br_2} \left( l \right) \nonumber \]
Solution:
Step 1: Plan the problem.
Break the reaction down into a net ionic equation and then into half-reactions. The substance that loses electrons is being oxidized and is the reducing agent. The substance that gains electrons is being reduced and is the oxidizing agent.
Step 2: Solve.
\[\begin{align*} \ce{Cl_2} \left( g \right) + \cancel{2 \ce{Na^+} \left( aq \right)} + 2 \ce{Br^-} \left( aq \right) &\rightarrow \cancel{2 \ce{Na^+} \left( aq \right)} + 2 \ce{Cl^-} \left( aq \right) + \ce{Br_2} \left( l \right) \\ \ce{Cl_2} \left( g \right) + 2 \ce{Br^-} \left( aq \right) &\rightarrow 2 \ce{Cl^-} \left( aq \right) + \ce{Br_2} \left(l \right) \: \: \: \: \: \left( \text{net ionic equation} \right) \end{align*} \nonumber \]
\[\begin{align*} &\text{Reduction:} \: \ce{Cl_2} \left( g \right) + 2 \ce{e^-} \rightarrow 2 \ce{Cl^-} \left( aq \right) \\ &\text{Oxidation:} \: 2 \ce{Br^-} \left( aq \right) \rightarrow \ce{Br_2} \left( l \right) + 2 \ce{e^-} \end{align*} \nonumber \]
The \(\ce{Cl_2}\) is being reduced and is the oxidizing agent. The \(\ce{Br^-}\) is being oxidized and is the reducing agent.
Write the following reaction in the form of half-equations. Identify each half-equation as an oxidation or a reduction. Also identify the oxidizing agent and the reducing agent in the overall reaction
\[\ce{Zn + 2Fe^{3+} -> Zn^{2+} +2Fe^{2+}} \nonumber \nonumber \]
Answer
The half-equations are
\(\ce{Zn -> Zn^{2+} + 2e^{-}}\) oxidation—loss of electrons
\(\ce{2e^{-} + 2Fe^{3+} -> 2Fe^{2+}}\) reduction—gain of electrons
Zinc has been oxidized, the oxidizing agent must have been the other reactant, namely, iron(III).
Iron(III) ion has been reduced, the zinc must be the reducing agent.
Identify the reducing and oxidizing agents in the balanced redox reaction:
\[ Cl_2 (aq) + 2Br^- (aq) \rightarrow 2Cl^- (aq) + Br_2 (aq)\nonumber \]
Oxidation half reaction
\[2 Br^- (aq) \rightarrow Br_2 (aq)\nonumber \]
Oxidation States: -1 to 0
Reduction Half Reaction
\[Cl_2 (aq) \rightarrow 2 Cl^- (aq)\nonumber \]
Oxidation States: 0 to -1
Overview
- Br- loses an electron; it is oxidized from Br- to Br2; thus, Br- is the reducing agent.
- Cl2 gains one electron; it is reduced from Cl2 to 2 Cl-; thus, Cl2 is the oxidizing agent.
Identify the oxidizing agent and the reducing agent in the following redox reaction:
\[MnO_4^- + SO_3^{2-} \rightarrow Mn^{2+} + SO_4^{2-}\nonumber \]
Solution
\(S\) is the reducing agent and \(Mn\) is the oxidizing agent.
Summary
- An oxidizing agent is a substance that causes oxidation by accepting electrons; therefore, it gets reduced.
- A reducing agent is a substance that causes reduction by losing electrons; therefore it gets oxidized.
- Examples of how to identify oxidizing and reducing agents are shown.
Contributors and Attributions
Ed Vitz (Kutztown University), John W. Moore (UW-Madison), Justin Shorb (Hope College), Xavier Prat-Resina (University of Minnesota Rochester), Tim Wendorff, and Adam Hahn.
- Diana Pearson, Connie Xu, Luvleen Brar (UCD)

