3.2: Serendipity in Science - X-Rays and Radioactivity
- Page ID
- 152148
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Understand the significance of the discovery of X-rays and radioactivity to the modern view of the structure of the atom.
The discovery of radioactivity took place over several years beginning with the discovery of x-rays in 1895 by Wilhelm Conrad Roentgen and continuing with such people as Henri Becquerel and the Curie family. The application of x-rays and radioactive materials is far reaching in medicine and industry. Radioactive material is used in everything from nuclear reactors to isotope infused saline solutions. These technologies allow us to utilize great amounts of energy and observe biological systems in ways which were unthinkable less than a century ago. As will be discussed in section3.4, the British physicist Ernest Rutherford (1871-1937) conducted experiments (based on the Curies' work) that led to the modern view of the structure of the atom.
Roentgen: The Discovery of X-Rays
From Section 3.1, we saw how J. J. Thomson discovered the electron by using cathode ray tubes (CRT). In November of 1895, a German physicist named Wilhelm Roentgen (Röntgen) used this same technology to discover the x-ray. Roentgen's ray originated from the CRT and reacted with a barium platinocyanide screen to fluoresce. After seeing this, Roentgen placed objects between this ray and the screen. He noted that the ray would still penetrate substances and leave an image on photographic film.
by using cathode ray tubes (CRT). In November of 1895, a German physicist named Wilhelm Roentgen (Röntgen) used this same technology to discover the x-ray. Roentgen's ray originated from the CRT and reacted with a barium platinocyanide screen to fluoresce. After seeing this, Roentgen placed objects between this ray and the screen. He noted that the ray would still penetrate substances and leave an image on photographic film.
When naming this type of radiation, Roentgen reflected back to his experiments. He could not confidently explain his observations. For this reason, he decided to call this new type of radiation the x-ray. Throughout his lifetime, he continue to explore this technology and even used his wife as a test subject (refer to chapter 1 for her x-ray image). By never patenting this invention, Roentgen freely shared the x-ray with other researchers and medical professionals. In 1901, he was awarded the Nobel Prize in physics for his work with the x-ray.
As with any new technology, people looked for ways to apply the x-ray to day to day life. Thomas Edison, the inventor of the light bulb, thought the average person should have an x-ray machine in their home. He designed x-ray machines to be smaller and portable. Unfortunately, many of his technicians died from radiation poisoning.

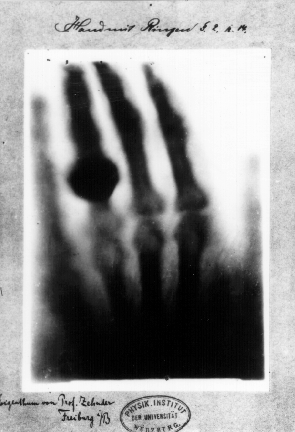
Hand mit Ringen (Hand with Rings): print of Wilhelm Röntgen's first "medical" X-ray, of his wife's hand, taken on 22 December 1895.
Figure \(\PageIndex{2}\): (left) Photo of Wilhelm Conrad Roengten, the discoverer of x-rays. (right) Surgical operation during World War I using a fluoroscope that used x-rays to find embedded bullets
During Edison's time, people would host x-ray parties in their homes. The host of these gatherings would allow guests to x-ray different parts of their body. People would leave these events with their framed souvenirs. X-rays were even used to measure foot size. Devices like the fluoroscope were placed in shoe stores. Technicians, consumers, and observers could look in oculars while the foot was being x-rayed. Unnecessary exposure to radiation was not considered as a hazard during these times. In this era, most believed this was the most accurate way to measure the foot.
In November 1896, an article entitled "the harmful effects of X-rays" was published in Nature. The witness was an X-ray demonstrator during the summer in London. He therefore paid for himself throughout the summer at the rate of several hours per day of exposure. He testified: "In the first two or three weeks I felt no inconvenience, but after a while appeared on the fingers of my right hand many dark spots which pierced under the skin, and gradually they became very painful; the rest of the skin was red and strongly inflamed My hand was so bad that I was constantly forced to bathe it in very cold water An ointment momentarily calm the pain but the epidermis had dried up had become hard and yellow like parchment and completely insensible, so I was not surprised when my hand began to peel. "
"Soon the skin and nails fall off and the fingers swelled, the pain remaining constant. I lost the skin of my right and left hands, and four of my nails have disappeared from the right hand and two left and three others were ready to fall off. During more than six weeks I was unable to hold anything in my right hand and I can not hold a pen since the loss of my nails …"
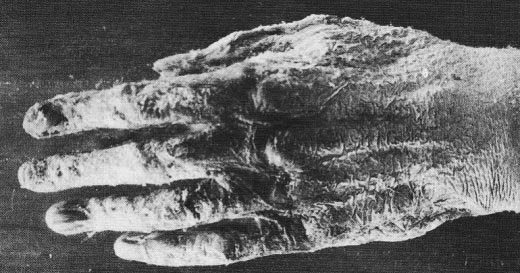
Figure \(\PageIndex{3}\): Radiation poisoning due to over exposure of x-rays.
Different applications use different parts of the X-ray spectrum.
Figure \(\PageIndex{4}\): X-rays are part of the electromagnetic spectrum, with wavelengths shorter than visible light.
The Discovery of Radioactivity
When Becquerel heard about Roentgen's discovery, he wondered if his fluorescent minerals would give the same x-rays. Becquerel placed some of his rock crystals on top of a well-covered photographic plate and sat them in the sunlight. The sunlight made the crystals glow with a bright fluorescent light, but when Becquerel developed the film he was very disappointed. He found that only one of his minerals, a uranium salt, had fogged the photographic plate. He decided to try again, and this time, to leave them out in the sun for a longer period of time. Fortunately, the weather did not cooperate and Becquerel 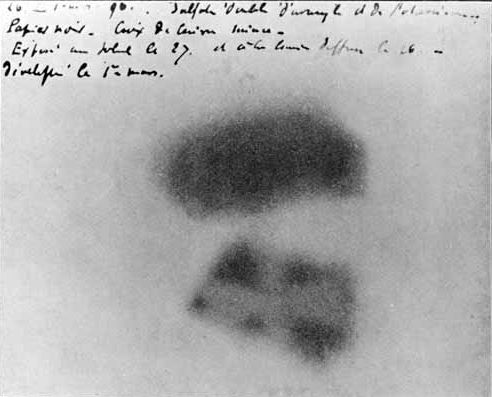 had to leave the crystals and film stored in a drawer for several cloudy days. Before continuing his experiments, Becquerel decided to check one of the photographic plates to make sure the chemicals were still good. To his amazement, he found that the plate had been exposed in spots where it had been near the uranium containing rocks and some of these rocks had not been exposed to sunlight at all. In later experiments, Becquerel confirmed that the radiation from the uranium had no connection with light or fluorescence, but the amount of radiation was directly proportional to the concentration of uranium in the rock. Becquerel had discovered radioactivity.
had to leave the crystals and film stored in a drawer for several cloudy days. Before continuing his experiments, Becquerel decided to check one of the photographic plates to make sure the chemicals were still good. To his amazement, he found that the plate had been exposed in spots where it had been near the uranium containing rocks and some of these rocks had not been exposed to sunlight at all. In later experiments, Becquerel confirmed that the radiation from the uranium had no connection with light or fluorescence, but the amount of radiation was directly proportional to the concentration of uranium in the rock. Becquerel had discovered radioactivity.
Figure \(\PageIndex{5}\): Image of Becquerel's photographic plate which has been fogged by exposure to radiation from a uranium salt. The shadow of a metal Maltese Cross placed between the plate and the uranium salt is clearly visible (Public Domain).
The Curies and Radium
One of Becquerel's assistants, a young Polish scientist named Maria Sklowdowska (to become Marie Curie after she married Pierre Curie), became interested in the phenomenon of radioactivity. With her husband, she decided to find out if chemicals other than uranium were radioactive. The Austrian government was happy to send the Curies a ton of pitchblende from the mining region of Joachimstahl because it was waste material that had to be disposed of anyway. The Curies wanted the pitchblende because it was the residue of uranium mining. From the ton of pitchblende, the Curies separated \(0.10 \: \text{g}\) of a previously unknown element, radium, in the form of the compound radium chloride. This radium was many times more radioactive than uranium.
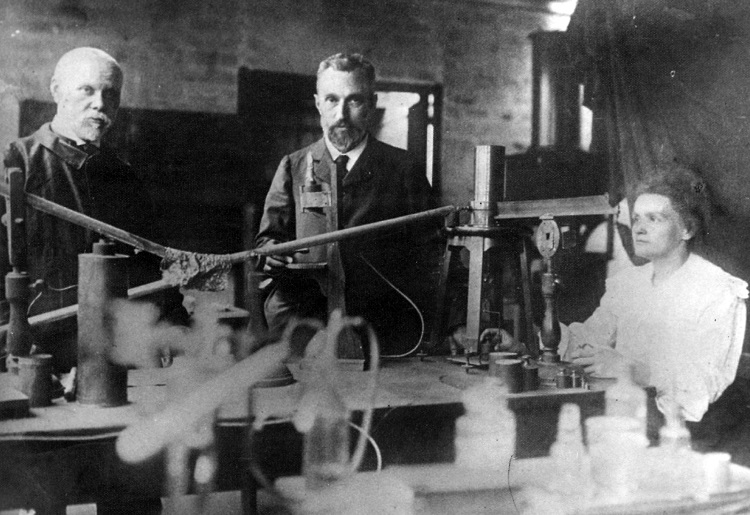
By 1902, the world was aware of a new phenomenon called radioactivity and of new elements which exhibited natural radioactivity. For this work, Becquerel and the Curies shared the 1903 Nobel Prize in Physics. These three researchers continued to work with hazardous radioactive materials. They experienced various ailments included burns and weakness by touching these substances. In 1906, Pierre Curie accidently fell under a horse drawn cart. He immediately died from his wounds and left Marie with two small children (Irene, 9 years old and Eve, 2 years old). After his death, Marie continued her research by identifying a new element, Polonium. In 1911, she was awarded the Nobel Prize in chemistry for the discoveries of radium and polonium. As of today, Marie Curie is the only person ever to have received two Nobel Prizes in the sciences.
Video\(\PageIndex{2}\): Everyone knows that winning the Nobel Prize is a big deal, but why do we even have a Nobel Prize? And why does it matter?
Summary
- Henri Becquerel, Marie Curie, and Pierre Curie shared the discovery of radioactivity.
- Wilhelm Roengtgen used cathode ray tubes to discover the x-ray.
Contributors and Attributions
- Wikibooks

