16.E: Organic Chemistry (Exercises)
- Page ID
- 65117
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Exercises (Hydrocarbons)
1. Define hydrocarbon. What are the two general types of hydrocarbons?
2. What are the three different types of aliphatic hydrocarbons? How are they defined?
3. Indicate whether each molecule is an aliphatic or an aromatic hydrocarbon; if aliphatic, identify the molecule as an alkane, an alkene, or an alkyne.
a. 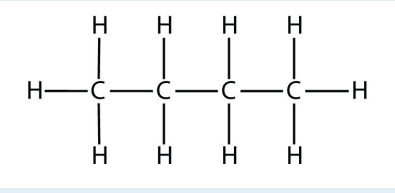
<smiles>CCCC</smiles>
b.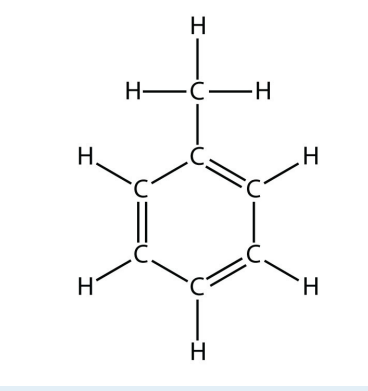
<smiles>Cc1ccccc1</smiles>
c.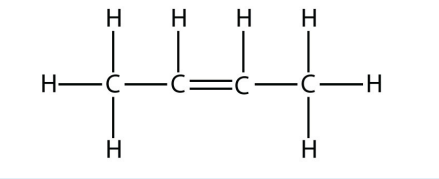
<smiles>C/C=C\C</smiles>
4. Indicate whether each molecule is an aliphatic or an aromatic hydrocarbon; if aliphatic, identify the molecule as an alkane, an alkene, or an alkyne.
a.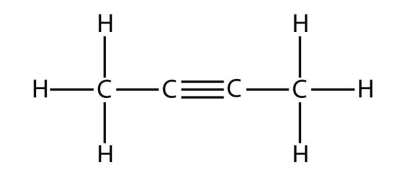
<smiles>CC#CC</smiles>
b.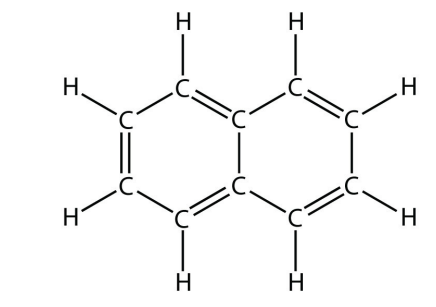
<smiles>c1ccc2ccccc2c1</smiles>
c. 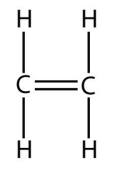
<smiles>C=C</smiles>
5. Indicate whether each molecule is an aliphatic or an aromatic hydrocarbon; if aliphatic, identify the molecule as an alkane, an alkene, or an alkyne.
a. 
<smiles>C1CC1</smiles>
b. 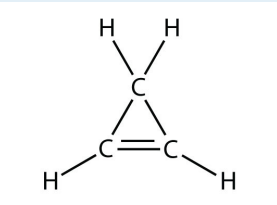
<smiles>C1=CC1</smiles>
c. 
<smiles>Cc1cccc(C)c1</smiles>
6. Indicate whether each molecule is an aliphatic or an aromatic hydrocarbon; if aliphatic, identify the molecule as an alkane, an alkene, or an alkyne.
a.
<smiles>C/C=C\C</smiles>
b. 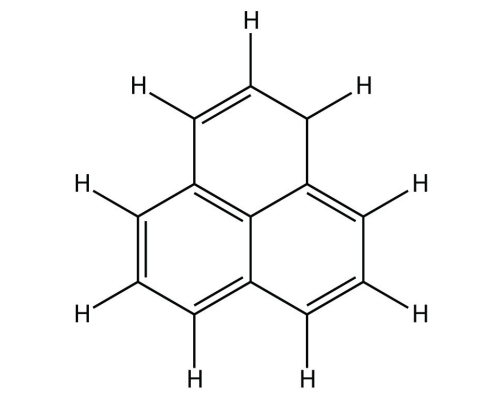
c. 
<smiles>C#CCC#C</smiles>
7. Name and draw the structural formulas for the four smallest alkanes.
8. Name and draw the structural formulas for the four smallest alkenes.
9. What does the term aromatic imply about an organic molecule?
10. What does the term normal imply when used for alkanes?
11. Explain why the name 1-propene is incorrect. What is the proper name for this molecule?
12. Explain why the name 3-butene is incorrect. What is the proper name for this molecule?
13. Name and draw the structural formula of each isomer of pentene.
14. Name and draw the structural formula of each isomer of hexyne.
15. Write a chemical equation for the reaction between methane and bromine.
16. Write a chemical equation for the reaction between ethane and chlorine.
17. Draw the structure of the product of the reaction of bromine with propene.
18. Draw the structure of the product of the reaction of chlorine with 2-butene.
19. Draw the structure of the product of the reaction of hydrogen with 1-butene.
20. Draw the structure of the product of the reaction of hydrogen with 2-pentene.
21. Write the balanced chemical equation for the combustion of heptane.
22. Write the balanced chemical equation for the combustion of nonane.
Answers
1. an organic compound composed of only carbon and hydrogen; aliphatic hydrocarbons and aromatic hydrocarbons
3.
a. aliphatic; alkane
b. aromatic
c. aliphatic; alkene
5.
a. aliphatic; alkane
b. aliphatic; alkene
c. aromatic
7.
a. 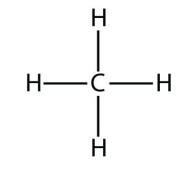 Methane
Methane
<smiles>C</smiles>
b. 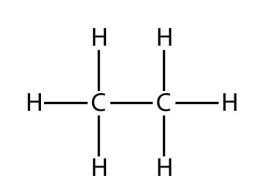 Ethane
Ethane
<smiles>CC</smiles>
c. 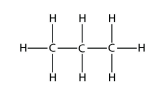 Propane
Propane
<smiles>CCC</smiles>
d. 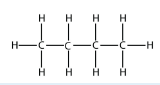 Butane
Butane
<smiles>CCCC</smiles>
9. Aromatic means that the molecule has a benzene ring.
11. The 1 is not necessary. The name of the compound is simply propene.
<smiles>C=CCCC</smiles>
13.
a. 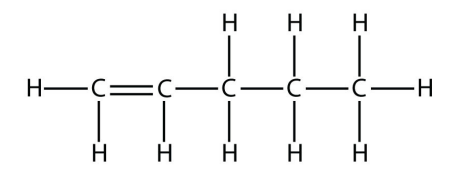 1-Pentene
1-Pentene
<smiles>C=CCCC</smiles>
b. 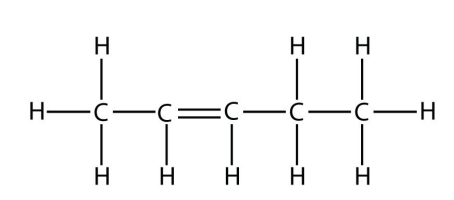 2-Pentene
2-Pentene
<smiles>CC=CCC</smiles>
15. \(\mathrm{CH}_4+\mathrm{Br}_2 \rightarrow \mathrm{CH}_3 \mathrm{Br}+\mathrm{HBr}\)
17. 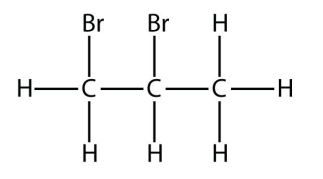
<smiles>CC(Br)CBr</smiles>
19. 
<smiles>CCCC</smiles>
21. \(\mathrm{C}_7 \mathrm{H}_{16}+11 \mathrm{O}_2 \rightarrow 7 \mathrm{CO}_2+8 \mathrm{H}_2 \mathrm{O}\)
Exercises (Branched Hydrocarbons)
1. How does a branched hydrocarbon differ from a normal hydrocarbon?
2. How does a substituent get its unique name?
3. Name this molecule.
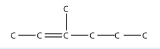
<smiles>CC=C(C)CCC</smiles>
4. Name this molecule.
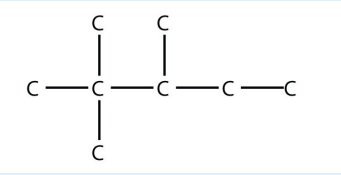
<smiles>CC(C)C(C)(C)C</smiles>
5. Name this molecule.

<smiles>C=CCC(C)(C)C</smiles>
6. Name this molecule.
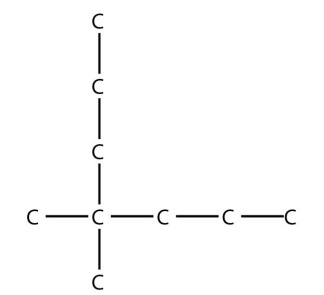
<smiles>CCCC(C)(C)CCCl</smiles>
7. Name this molecule.
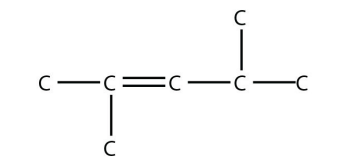
<smiles>CC(C)=CC(C)C</smiles>
8. Name this molecule.
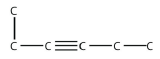
<smiles>CCC#CCC</smiles>
9. Name this molecule.
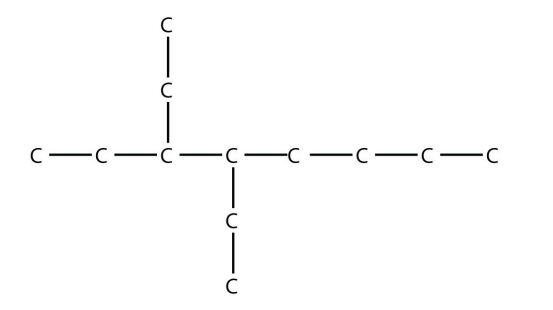
<smiles>CCCCC(CC)C(CC)CC</smiles>
10. Name this molecule.
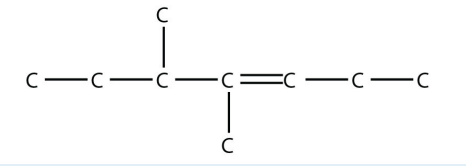
<smiles>CCC=C(C)C(C)CC</smiles>
11. Name this molecule.
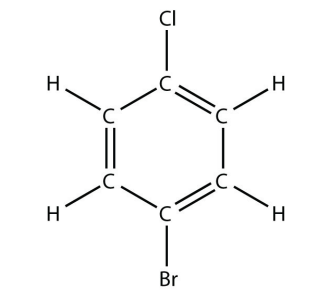
<smiles>Clc1ccccc1</smiles>
12. Name this molecule.
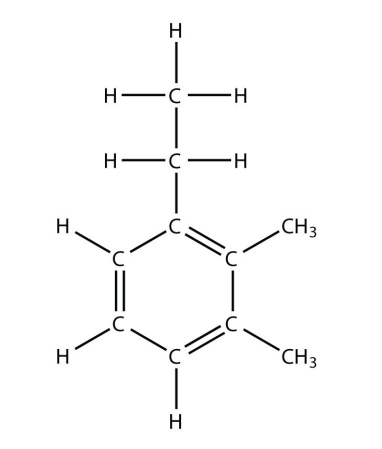
<smiles>CCCc1ccccc1C</smiles>
13. Draw the carbon backbone for each molecule.
a. 3,4-diethyloctane
b. 2,2-dimethyl-4-propylnonane
14. Draw the carbon backbone for each molecule.
a. 3-ethyl-4-methyl-3-heptene
b. 3,3-diethyl-1-pentyne
15. Draw the carbon backbone for each molecule.
a. 4-ethyl-4-propyl-2-octyne
b. 5-butyl-2,2-dimethyldecane
16. Draw the carbon backbone for each molecule.
a. 3,4-diethyl-1-hexyne
b. 4-propyl-3-ethyl-2-methyloctane
17. The name 2-ethylhexane is incorrect. Draw the carbon backbone and write the correct name for this molecule.
18. The name 3-butyl-7-methyloctane is incorrect. Draw the carbon backbone and write the correct name for this molecule.
Answers
1. A branched hydrocarbon does not have all of its \(\mathrm{C}\) atoms in a single row.
3. 3-methyl-2-hexene
5. 4,4-dimethyl-1-pentene
7. 2,4-dimethyl-2-pentene
9. 3,4-diethyloctane
11. 1-bromo-4-chlorobenzene
13.
a. 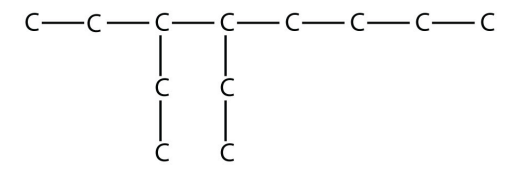
<smiles>CCCCC(CC)C(CC)CC</smiles>
b. 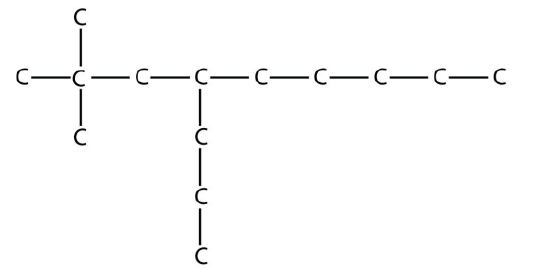
<smiles>CCCCCC(CCC)CC(C)(C)C</smiles>
15. a. 
<smiles>CC#CC(CC)(CCC)CCCCC</smiles>
b. 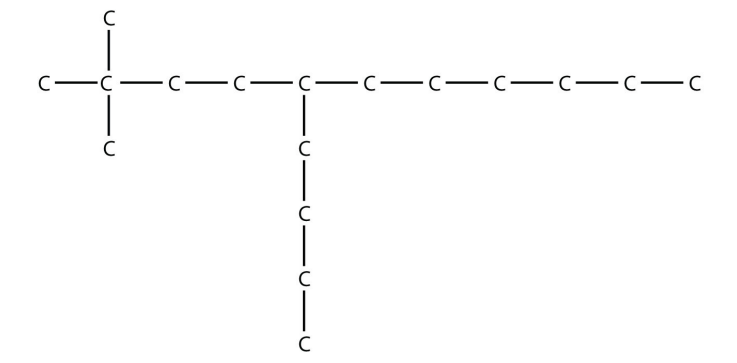
<smiles>CCCCCCC(CCCC)CCC(C)(C)C</smiles>
17. 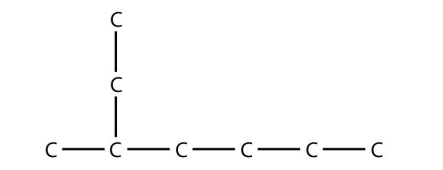 2-methylheptane
2-methylheptane
<smiles>CCCCC(C)CC</smiles>
Exercises (Alkyl Halides and Alcohols)
1. Define functional group and give two examples.
2. What is elimination? How does it differ for alkyl halides and alcohols?
3. Name this molecule.

<smiles>CC(C)Br</smiles>
4. Name this molecule.
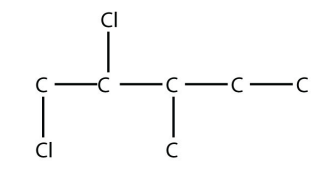
<smiles>CCC(C)C(Cl)CCl</smiles>
5. Name this molecule.
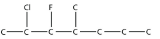
<smiles>CCCC(C)C(F)C(C)Cl</smiles>
6. Name this molecule.
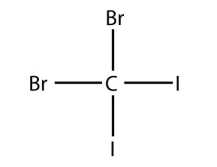
<smiles>BrC(Br)(I)I</smiles>
7. Name this molecule.

<smiles>CC(C)(C)O</smiles>
8. Name this molecule.
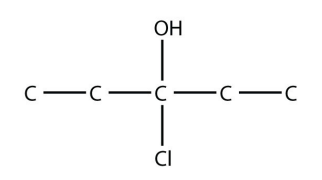
<smiles>CCC(O)(Cl)CC</smiles>
9. Name this molecule.
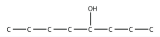
<smiles>CCCCC(O)CCCl</smiles>
10. Name this molecule.
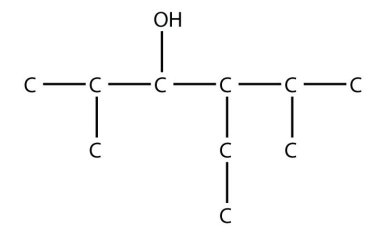
<smiles>CCC(C(C)C)C(O)C(C)C</smiles>
11. Predict the product(s) of this elimination reaction.
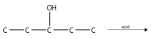
<smiles>CCC(O)CC</smiles>
12. Predict the product(s) of this elimination reaction.
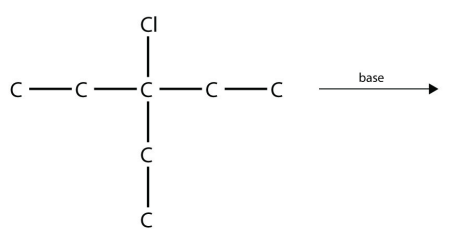
<smiles>CCC(Cl)(CC)CC</smiles>
13. Predict the product(s) of this elimination reaction.
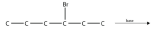
<smiles>CCCC(Br)CC</smiles>
14. Predict the product(s) of this elimination reaction.

<smiles>CCC(C)(O)CC</smiles>
Answers
1. a group of atoms with a certain reactivity; halogen atoms and alcohol groups (answers will vary).
3. 2-bromobutane
5. 2-chloro-3-fluoro-4-methylheptane
7. 2-methyl-2-propanol
9. 4-octanol
11. 2-pentene
13. 2-hexene and 3-hexene
Exercises (Other Oxygen-Containing Functional Groups)
1. Name a similarity between the functional groups found in aldehydes and ketones. Can you name a difference between them?
2. Explain how a carboxylic acid is used to make an ester.
3. Name each molecule.
a. 
<smiles>CCC=O</smiles>
b. 
<smiles>CCC(=O)Cl</smiles>
4. Name each molecule.
a. 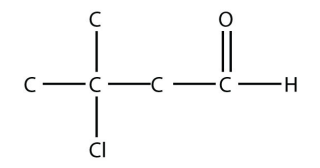
<smiles>CC(C)(Cl)CC=O</smiles>
b. 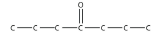
<smiles>CCCC(=O)CCC</smiles>
5. Name each molecule.
a. 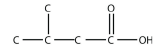
<smiles>CC(C)CC(=O)O</smiles>
b. 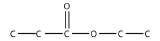
<smiles>CCC(=O)OCCl</smiles>
6. Name each molecule.
a. 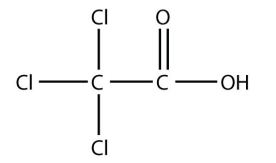
<smiles>O=C(O)C(Cl)(Cl)Cl</smiles>
b.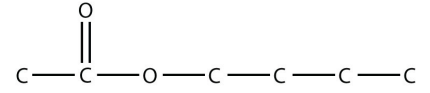
<smiles>CCCCOC(C)=O</smiles>
7. Name this molecule.
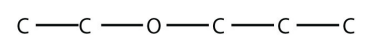
8. Name this molecule.

<smiles>CCOc1ccccc1</smiles>
9. Give an alternate but acceptable name to the molecule in Exercise 3b.
10. Give an alternate but acceptable name to the molecule in Exercise 4 b.
11. Complete this chemical reaction.
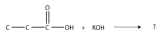
<smiles>CCC(=O)[OH2+]</smiles>
12. Complete this chemical reaction.
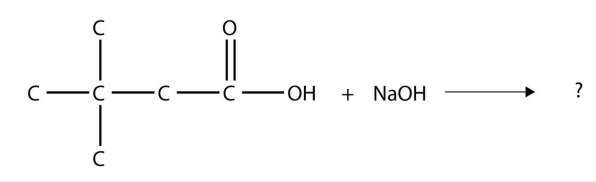
<smiles>CC(C)(C)CC(=O)O</smiles>
13. The drug known as aspirin has this molecular structure:
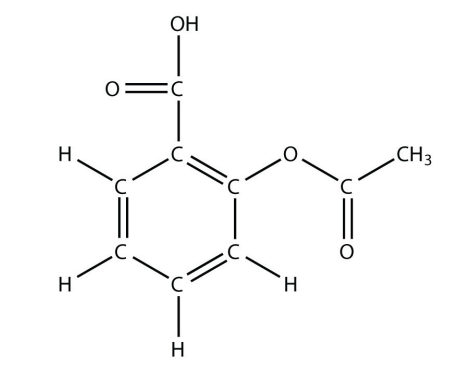
<smiles>CC(=O)Oc1ccccc1</smiles>
Identify the functional group(s) in this molecule.
14. The drug known as naproxen sodium is the sodium salt of this molecule:
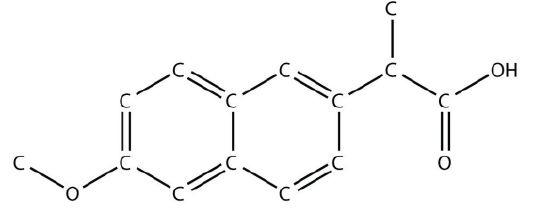
<smiles>COc1ccc2cc(C(C)C(=O)O)ccc2c1</smiles>
(The extra \(\mathrm{H}\) atoms are omitted for clarity.) Identify the functional group(s) i this molecule.
15. Identify the ester made by reacting these molecules.

<smiles>CCC(=O)[OH2+]</smiles>
16. Identify the ester made by reacting these molecules.
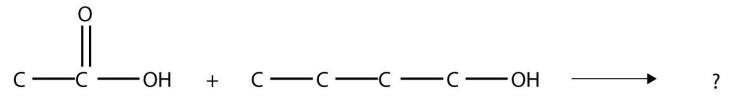
Answers
1. They both have a carbonyl group, but an aldehyde has the carbonyl group at the end of a carbon chain, and a ketone has the carbonyl group in the middle.
3. a. propanal
b. 2-butanone
5. a. 3-methylbutanoic acid
b. ethyl propionate
7. ethyl propyl ether
9. ethyl methyl ketone
11. \(\mathrm{H}_2 \mathrm{O}+\mathrm{KCH}_3 \mathrm{CH}_2 \mathrm{CO}_2\)
13. acid, ester, and aromatic (benzene ring)
15. propyl propionate
Exercises (Other Functional Groups)
1. What are the structure and name of the smallest amine?
2. What are the structure and name of the smallest thiol?
3. Identify each compound as a primary, secondary, or tertiary amine.
a. 
<smiles>NC(CS)C(=O)O</smiles>
b. 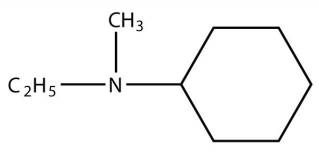
<smiles>CCN(C)C1CCCCC1</smiles>
c. 
<smiles>CCNC</smiles>
4. Identify each compound as a primary, secondary, or tertiary amine.
a. 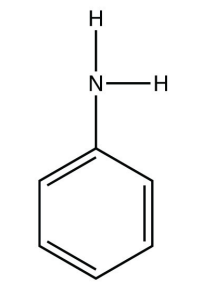
<smiles>Nc1ccccc1</smiles>
b. 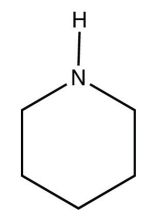
<smiles>C1CCNCC1</smiles>
c. 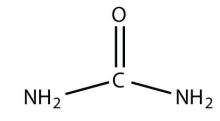
<smiles>NC(N)=O</smiles>
5. Write the chemical reaction between each amine in Exercise 3 and \(\mathrm{HCl}\).
6. Write the chemical reaction between each amine in Exercise 4 and \(\mathrm{HNO}_3\).
7. Name each amine.
a. 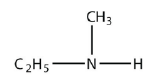
<smiles>CCNC</smiles>
b. 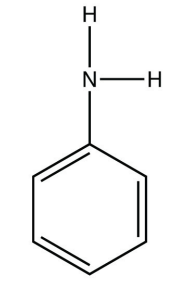
<smiles>Nc1ccccc1</smiles>
8. Name each amine.
a. 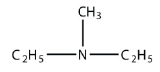
<smiles>CCN(C)CC</smiles>
b. 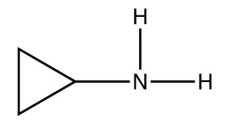
<smiles>CNC1CC1</smiles>
9. A peptide is a short chain of amino acids connected by amide bonds. How many amide bonds are present in this peptide?
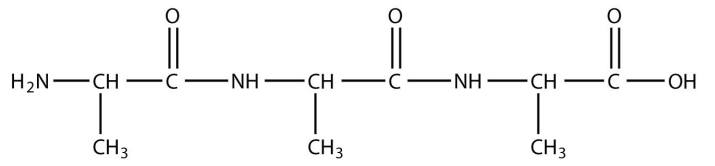
<smiles>CC(N)C(=O)NC(C)C(=O)NC(C)C(=O)O</smiles>
10. How many amide bonds are present in this peptide? (See Exercise 9 for the definition of a peptide.)
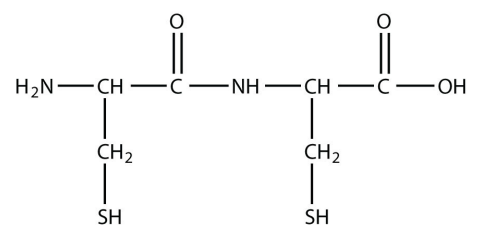
<smiles>NC(CS)C(=O)NC(CS)C(=O)O</smiles>
11. Draw the backbone structure of the amide formed by reacting propylamine with propanoic acid.
12. Draw the backbone structure of the amide formed by reacting hexylamine with ethanoic acid.
13. Name each thiol using the -thiol suffix.
a. 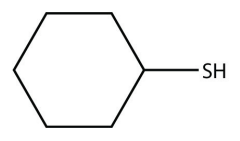
b. \(\mathrm{C}_4 \mathrm{H}_9-\mathrm{SH}\)
14. Name each thiol in Exercise 13 with the mercaptan label.
15. One component of skunk spray is 3-methyl-1-butanethiol. Draw its structure. (The 1 indicates the position of the \(\mathrm{S}\) atom.)
16. An S-S bond can be fairly easily broken into proteins, yielding two lone cysteine units in a protein chain. Is this process an oxidation or a reduction? Explain your answer.
Answers
1. \(\mathrm{CH}_3 \mathrm{NH}_2 ;\) methylamine
3.
a. primary
b. tertiary
c. secondary
5.
a. \(\mathrm{C}_3 \mathrm{H}_3 \mathrm{CO}_2 \mathrm{HSHNH}_2+\mathrm{HCl} \rightarrow \mathrm{C}_3 \mathrm{H}_3 \mathrm{CO}_2 \mathrm{HSHNH}_3 \mathrm{Cl}\)
b. \(\left(\mathrm{C}_6 \mathrm{H}_{11}\right)\left(\mathrm{C}_2 \mathrm{H}_5\right)\left(\mathrm{CH}_3\right) \mathrm{N}+\mathrm{HCl} \rightarrow\left(\mathrm{C}_6 \mathrm{H}_{11}\right)\left(\mathrm{C}_2 \mathrm{H}_5\right)\left(\mathrm{CH}_3\right) \mathrm{NHCl}\)
c. \(\left(\mathrm{C}_2 \mathrm{H}_5\right)\left(\mathrm{CH}_3\right) \mathrm{NH}+\mathrm{HCl} \rightarrow\left(\mathrm{C}_2 \mathrm{H}_5\right)\left(\mathrm{CH}_3\right) \mathrm{NH}_2 \mathrm{Cl}\)
7.
a. ethylmethylamine
b. phenylamine
9. two
11. 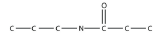
<smiles>CCCNC(=O)CC</smiles>
13.
a. cyclohexanethiol
b. butanethiol
15. 
<smiles>CC(C)CCS</smiles>
Exercises (Polymers)
1. Explain the relationship between a monomer and a polymer.
2. Must a monomer have a double bond to make a polymer? Give an example to illustrate your answer.
3. Draw the polymer made from this monomer.
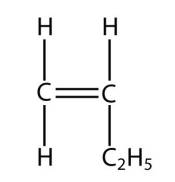
<smiles>C=CCC</smiles>
4. Draw the polymer made from this monomer.
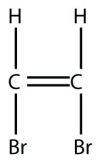
<smiles>Br/C=C/Br</smiles>
5. What is the difference between an addition polymer and a condensation polymer?
6. What is the difference between a condensation polymer and a copolymer?
7. List three properties of polymers that vary widely with composition.
8. List three uses of polymers.
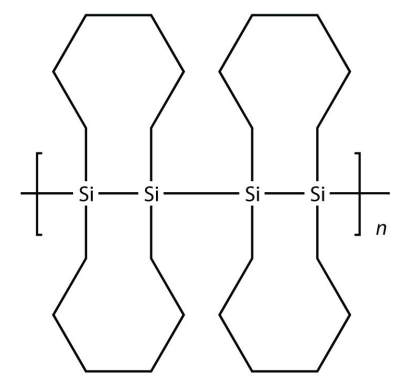
9. Draw the silicone made from this monomer.
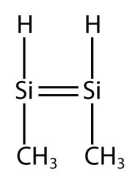
<smiles>C[SiH]=[SiH]</smiles>
10. Draw the silicone made from this monomer.

11. Explain how starch is a polymer.
12. What is the difference between starch and cellulose?
13. Explain how protein is a polymer.
14. What are the parts that compose DNA?
Answers
1. A polymer is many monomers bonded together.
3. 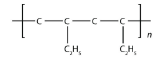
<smiles>CCC(CCC(C)(C)CC)C(C)(C)C</smiles>
5. In an addition polymer, no small molecule is given off as a product, whereas in a condensation polymer, small parts of each monomer come off as a small molecule.
7. solubility in \(\mathrm{H}_2 \mathrm{O}\) and other solvents, melting point, flammability, color, hardness, transparency, film thickness, wetability, surface friction, moldability, and particle size (answers will vary)
9. 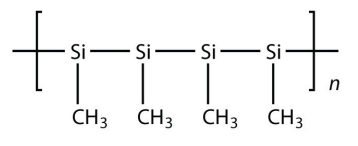
<smiles>C[SiH](C)[SiH](C)[SiH](C)[SiH](C)C</smiles>
11. Starch is composed of many glucose monomer units.
13. Proteins are polymers of amino acids, which act as the monomers.
Additional Exercises
1. Cycloalkanes are named based on the number of \(C\) atoms in them, just like regular alkanes, but with the prefix cyclo- on the name. What are the names of the three smallest cycloalkanes?
2. Cycloalkenes are named similarly to cycloalkanes (see Exercise 1). What are the names of the cycloalkenes with five, six, and seven C atoms?
3. Draw the carbon backbone of all noncyclic alkanes with only four \(\mathrm{C}\) atoms.
4. Draw the carbon backbone of all noncyclic alkanes with only five \(\mathrm{C}\) atoms.
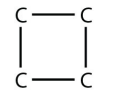
5. Cyclic alkanes can also have substituent groups on the ring. Draw the carbon backbone of all cyclic alkanes with only four C atoms.
6. Cyclic alkanes can also have substituent groups on the ring. Draw the carbon backbone of all cyclic alkanes with only five \(\mathrm{C}\) atoms.
7. Draw and name all possible isomers of pentene.
8. Draw and name all possible normal (that is, straight-chain) isomers of heptyne.
9. Polyunsaturated alkenes have more than one \(\mathrm{C}-\mathrm{C}\) double bond. Draw the carbon backbone of all possible noncyclic polyunsaturated alkenes with four C atoms and two double bonds. What are the complete molecular formulas for each possible molecule?
10. Draw the carbon backbone of all possible five-carbon cyclic alkenes with two double bonds, assuming no substituents on the ring.
11. If a hydrocarbon is combined with enough halogen, all the \(\mathrm{H}\) atoms will eventually be substituted with that halogen atom. Write the balanced chemical reaction between ethane and excess chlorine.
12. If a hydrocarbon is combined with enough halogen, all the \(\mathrm{H}\) atoms will eventually be substituted with that halogen atom. Write the balanced chemical reaction between butane and excess bromine.
13. Molecules with multiple double bonds can also participate in addition reactions. Draw the structure of the product when butadiene, \(\mathrm{CH}_2=\mathrm{CH}-\mathrm{CH}=\mathrm{CH}_2\), reacts with chlorine.
14. Molecules with multiple double bonds can also participate in addition reactions. Draw the structure of the product when allene, \(\mathrm{CH}_2=\mathrm{C}=\mathrm{CH}_2\), reacts with bromine.
15. What is the maximum number of methyl groups that can be on a propane backbone before the molecule cannot be named as a propane compound?
16. Explain why cycloethane cannot exist as a real molecule.
17. In the gasoline industry, what is called isooctane is actually 2,2,4-trimethylpentane. Draw the structure of isooctane.
18. Isooctane (see Exercise 17) is an isomer of what straight-chain alkane?
19. The actual name for the explosive TNT is 2,4,6-trinitrotoluene. If the structure of TNT is
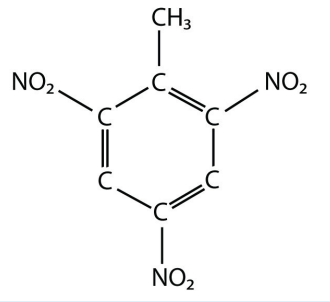
<smiles>Cc1c([N+](=O)[O-])cc([N+](=O)[O-])cc1[N+](=O)[O-]</smiles>
propose the structure of the parent compound toluene.
20. Phenol is hydroxybenzene, the simplest aromatic alcohol. Picric acid is an explosive derivative of phenol whose formal name is 2,4,6-trinitrophenol. With reference to Exercise 19, draw the structure of picric acid.
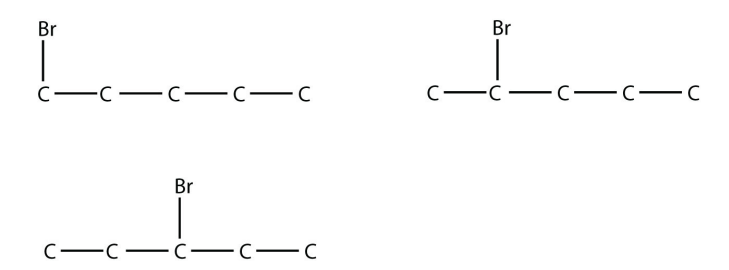
21. Draw the structures of all possible straight-chain isomers of bromopentane.
22. Draw the structures of all the possible isomers of butanol. Include branched isomers.
23. What is the final product of the double elimination of \(\mathrm{HCl}\) from 1,1-dichloroethane?
24. Draw the structure of the final product of the double elimination of 1,3-dibromopropane.
25. Draw the structure and name of the alcohol whose double elimination would yield the same product as in Exercise 23. Name the molecule as a hydroxylsubstituted compound.
26. Draw the structure and name of the alcohol whose double elimination would yield the same product as in Exercise 24. Name the molecule as a hydroxylsubstituted compound.
27. Draw the smallest molecule that can have a separate aldehyde and carboxylic acid group.
28. Name the functional group(s) in urea, a molecule with the following structure:
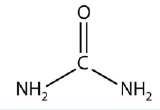
<smiles>NC(N)=O</smiles>
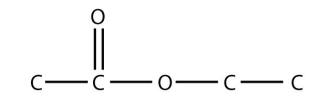
29. Ethyl acetate is a common ingredient in nail-polish remover because it is a good solvent. Draw the structure of ethyl acetate.
30. A lactone is an ester that has its ester functional group in a ring. Draw the structure of the smallest possible lactone (which is called acetolactone, which might give you a hint about its structure).
31. Draw the structure of diethyl ether, once used as an anesthetic.
32. The smallest cyclic ether is called an epoxide. Draw its structure.
33. The odor of fish is caused by the release of small amine molecules, which vaporize easily and are detected by the nose. Lemon juice contains acids that react with the amines and make them not as easily vaporized, which is one reason why adding lemon juice to seafood is so popular. Write the chemical reaction of \(\mathrm{HCl}\) with trimethylamine, an amine that is given off by seafood.
34. Putrescine and cadaverine are molecules with two amine groups on the opposite ends of a butane backbone and a pentane backbone, respectively. They are both emitted by rotting corpses. Draw their structures and determine their molecular formulas.
35. With four monomers, draw two possible structures of a copolymer composed of ethylene and propylene.
36. With four monomers, draw two possible structures of a copolymer composed of ethylene and styrene.
37. Draw the silicone that can be made from this monomer:
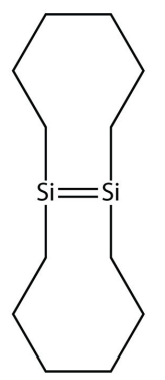
38. One of the ingredients in the original Silly Putty was a silicone polymer with two methyl groups on each Si atom. Draw this silicone.
Answers
1. cyclopropane, cyclobutane, and cyclopentane
3.

<smiles>CC(C)C</smiles>
5. 
<smiles>C1CCC1</smiles> <smiles>CC1CC1</smiles>
7. 
9. 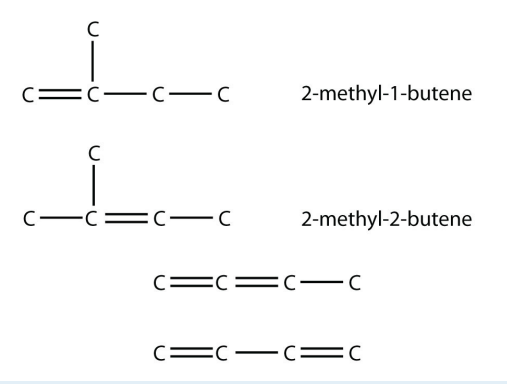
Both molecular formulas are \(\mathrm{C}_4 \mathrm{H}_6\).
11. \(\mathrm{C}_2 \mathrm{H}_6+6 \mathrm{Cl}_2 \rightarrow \mathrm{C}_2 \mathrm{Cl}_6+6 \mathrm{HCl}\)
13. 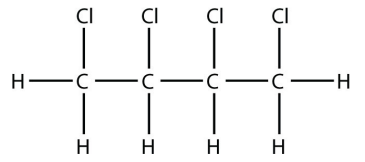
<smiles>CCl</smiles>
15. two
17. 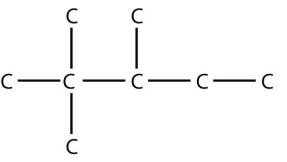
<smiles>CCC(C)C(C)(C)C</smiles>
19. 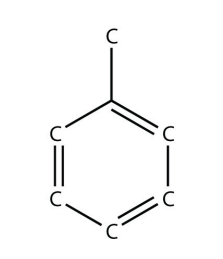
<smiles>CCCCCBr</smiles> <smiles>CCCC(C)Br</smiles>
21. 
<smiles>CCC(Br)CC</smiles>
23. ethyne

<smiles>OCCO</smiles>
or

<smiles>OC(O)Cl</smiles>
25. The names are 1,2-dihydroxyethane and 1,1-dihydroxyethane, respectively.
27. 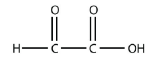
<smiles>O=CC(=O)O</smiles>
29. 
<smiles>CCOC(C)=O</smiles>
31. 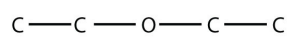
33. \(\left(\mathrm{CH}_3\right)_3 \mathrm{~N}+\mathrm{HCl} \rightarrow\left(\mathrm{CH}_3\right)_3 \mathrm{NHCl}\)
35. (answers will vary)
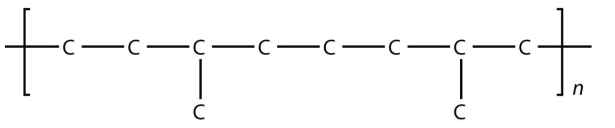
<smiles>CC(CCCCC(C)(C)C)CCC(C)(C)C</smiles>
or

<smiles>CC(CCCCC(C)CC(C)(C)C)CC(C)(C)C</smiles>
37.