15.11: Physical Properties of Amines
- Page ID
- 16034
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Explain why the boiling points of primary and secondary amines are higher than those of alkanes or ethers of similar molar mass but are lower than those of alcohols.
- Compare the boiling points of tertiary amines with alcohols, alkanes, and ethers of similar molar mass.
- Compare the solubilities in water of amines of five or fewer carbon atoms with the solubilities of comparable alkanes and alcohols in water.
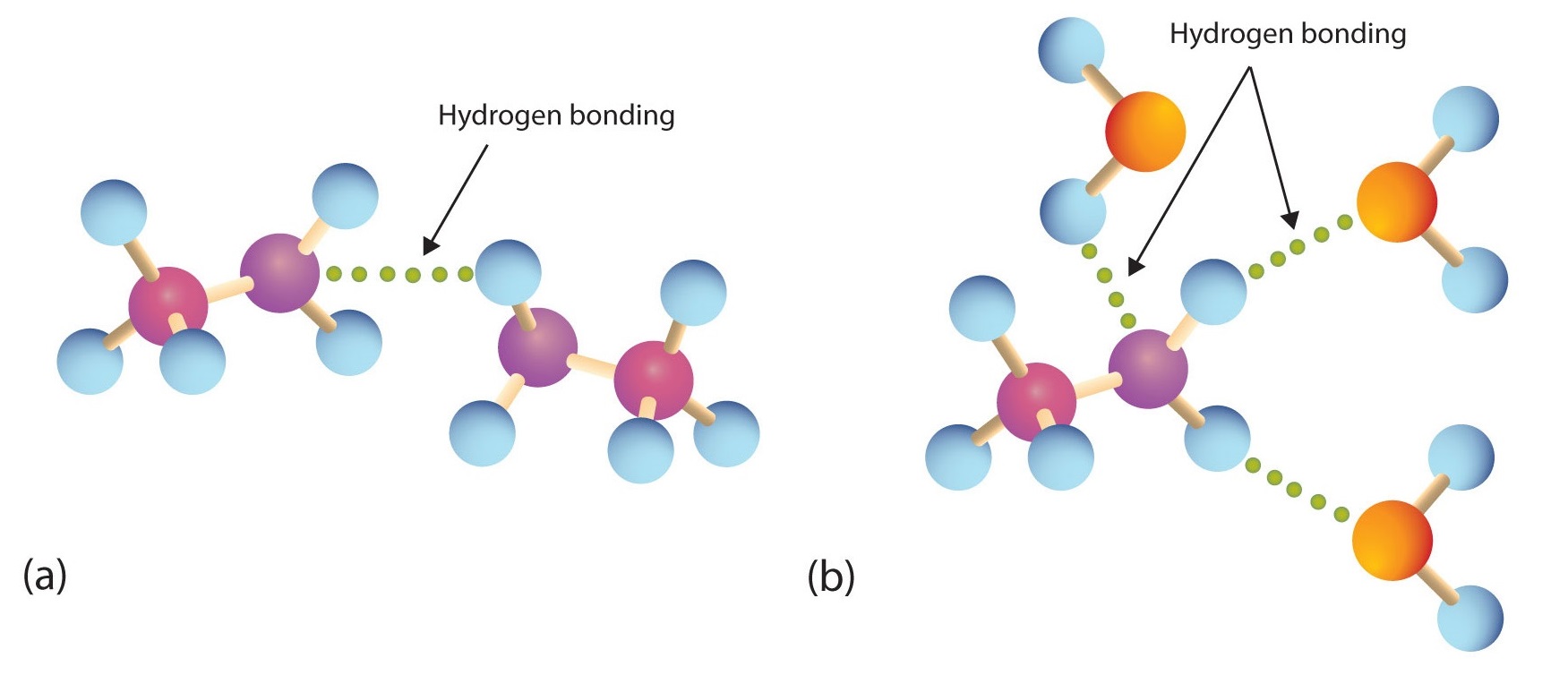
Primary and secondary amines have hydrogen atoms bonded to an nitrogen atom and are therefore capable of hydrogen bonding (part (a) of Figure \(\PageIndex{1}\)), although not as strongly as alcohol molecules (which have hydrogen atoms bonded to an oxygen atom, which is more electronegative than nitrogen). These amines boil at higher temperatures than alkanes but at lower temperatures than alcohols of comparable molar mass. For example, compare the boiling point of methylamine (CH3NH2; −6°C) with those of ethane (CH3CH3; −89°C) and methanol (CH3OH; 65°C). Tertiary amines have no hydrogen atom bonded to the nitrogen atom and so cannot participate in intermolecular hydrogen bonding. They have boiling points comparable to those of ethers (Table \(\PageIndex{1}\)).
| Name | Condensed Structural Formula | Class | Molar Mass | Boiling Point (°C) | Solubility at 25°C (g/100 g Water) |
|---|---|---|---|---|---|
| butylamine | CH3CH2CH2CH2NH2 | 1° | 73 | 78 | miscible |
| diethylamine | (CH3CH2)2NH | 2° | 73 | 55 | miscible |
| butyl alcohol | CH3CH2CH2CH2OH | — | 74 | 118 | 8 |
| dipropylamine | (CH3CH2CH2)2NH | 2° | 101 | 111 | 4 |
| triethylamine | (CH3CH2)3N | 3° | 101 | 90 | 14 |
| dipropyl ether | (CH3CH2CH2)2O | — | 102 | 91 | 0.25 |
All three classes of amines can engage in hydrogen bonding with water (Figure \(\PageIndex{1b}\)). Amines of low molar mass are quite soluble in water; the borderline of solubility in water is at five or six carbon atoms.
Amines have “interesting” odors. The simple ones smell very much like ammonia. Higher aliphatic amines smell like decaying fish. Or perhaps we should put it the other way around: Decaying fish give off odorous amines. The stench of rotting fish is due in part to two diamines: putrescine and cadaverine. They arise from the decarboxylation of ornithine and lysine, respectively, amino acids that are found in animal cells.
\[\ce{HOCH2CH2OH} \nonumber \]
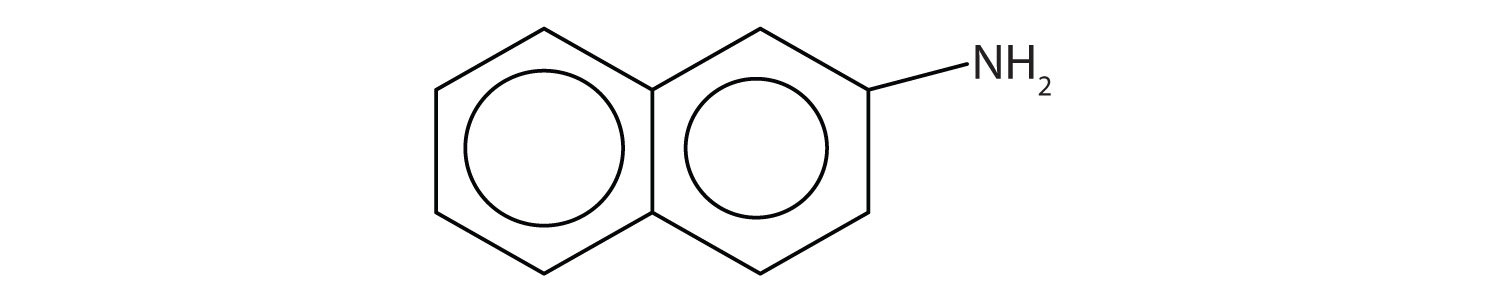
Aromatic amines generally are quite toxic. They are readily absorbed through the skin, and workers must exercise caution when handling these compounds. Several aromatic amines, including β-naphthylamine, are potent carcinogens.
Key Takeaways
- Primary and secondary amines have higher boiling points than those of alkanes or ethers of similar molar mass because they can engage in intermolecular hydrogen bonding. Their boiling points are lower than those of alcohols because alcohol molecules have hydrogen atoms bonded to an oxygen atom, which is more electronegative.
- The boiling points of tertiary amines, which cannot engage in hydrogen bonding because they have no hydrogen atom on the nitrogen atom, are comparable to those of alkanes and ethers of similar molar mass.
- Because all three classes of amines can engage in hydrogen bonding with water, amines of low molar mass are quite soluble in water.


