Introduction to Transition Metals I
- Page ID
- 19706
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The elements of the second and third rows of the Periodic Table show gradual changes in properties across the table from left to right as expected. Electrons in the outer shells of the atoms of these elements have little shielding effects resulting in an increase in effective nuclear charge due to the addition of protons in the nucleus. Consequently, the effects on atomic properties are: smaller atomic radius, increased first ionization energy, enhanced electronegativity and more nonmetallic character. This trend continues until one reaches calcium (Z=20). There is an abrupt break at this point. The next ten elements called the first transition series are remarkably similar in their physical and chemical properties. This general similarity in properties has been explained in terms of their relatively small difference in effective nuclear charge over the series. This occurs because each additional electron enters the penultimate 3d shell providing an effective shield between the nucleus and the outer 4s shell.
Thus, the transition elements can be defined as those in which the d electron shells are being filled and so we generally ignore Sc and Zn where Sc(III) is d0 and Zn(II) is d10.
Physical Properties
It is useful, at the beginning, to identify the physical and chemical properties of transition elements which differ from main group elements (s-block) such as Calcium. Transition elements:
- have large charge/radius ratio;
- are hard and have high densities;
- have high melting and boiling points;
- form compounds which are often paramagnetic;
- show variable oxidation states;
- form coloured ions and compounds;
- form compounds with profound catalytic activity;
- form stable complexes.
| Element | Group | density /g cm-3 |
m. p. / °C |
b.p. / °C |
radius / pm |
free atom configuration |
ionization energy / kJ mol-1 |
Uses |
|---|---|---|---|---|---|---|---|---|
| Sc | 3 | 2.99 | 1541 | 2831 | 164 | [Ar] 3d14s2 | 631 | |
| Ti | 4 | 4.50 | 1660 | 3287 | 147 | [Ar]3d24s2 | 658 | -engines/aircraft industry-density is 60% of iron |
| V | 5 | 5.96 | 1890 | 3380 | 135 | [Ar]3d34s2 | 650 | -stainless steel, 19% Cr, 9% Ni the rest Fe |
| Cr | 6 | 7.20 | 1857 | 2670 | 129 | [Ar]3d54s1 | 653 | -alloys eg with C steel, the most significant use |
| Mn | 7 | 7.20 | 1244 | 1962 | 137 | [Ar]3d54s2 | 717 | -alloys eg with Cu |
| Fe | 8 | 7.86 | 1535 | 2750 | 126 | [Ar]3d64s2 | 759 | -alloys eg with C steel, the most significant use |
| Co | 9 | 8.90 | 1495 | 2870 | 125 | [Ar]3d74s2 | 758 | -alloys eg with Cr and W for hardened drill bits |
| Ni | 10 | 8.90 | 1455 | 2730 | 125 | [Ar]3d84s2 | 737 | -alloys Fe/Ni armor plating, resists corrosion |
| Cu | 11 | 8.92 | 1083 | 2567 | 128 | [Ar]3d104s1 | 746 | -high electrical conductivity (2nd to Ag), wiring |
| Zn | 12 | 7.14 | 420 | 907 | 137 | [Ar]3d104s2 | 906 |
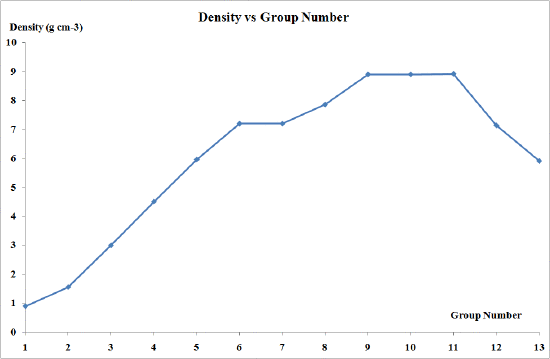
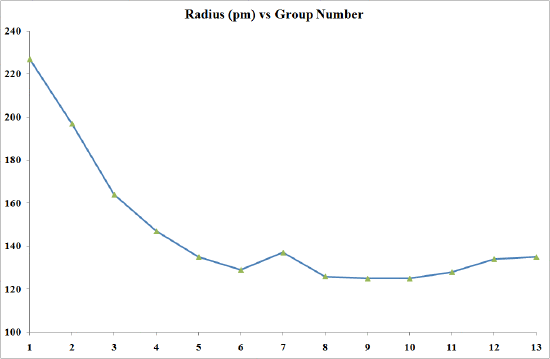
Densities and metallic radii
The transition elements are much denser than the s-block elements and show a gradual increase in density from scandium to copper. This trend in density can be explained by the small and irregular decrease in metallic radii coupled with the relative increase in atomic mass.
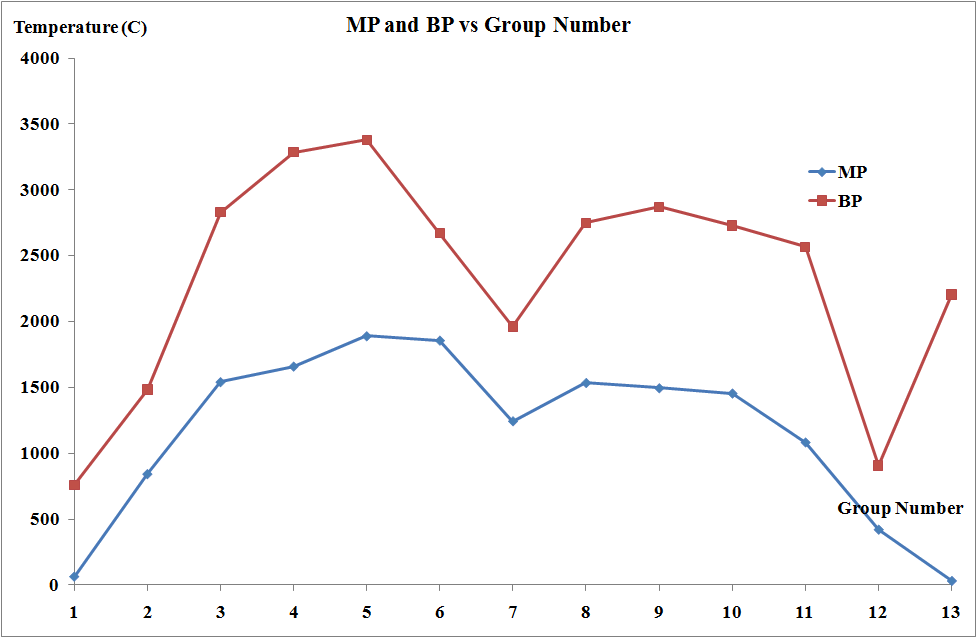
Melting and boiling points
The melting points and the molar enthalpies of fusion of the transition metals are both high in comparison to main group elements. This arises from strong metallic bonding in transition metals which occurs due to delocalization of electrons facilitated by the availability of both d and s electrons.
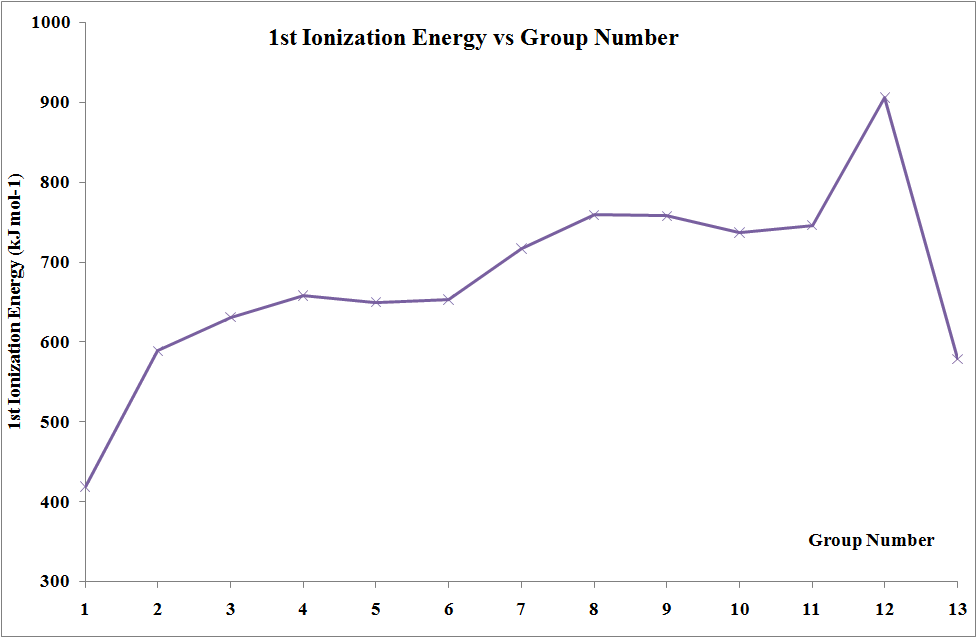
Ionization Energies
In moving across the series of metals from scandium to zinc a small change in the values of the first and second ionization energies is observed. This is due to the build-up of electrons in the immediately underlying d-sub-shells that efficiently shields the 4s electrons from the nucleus and minimizing the increase in effective nuclear charge from element to element. The increases in third and fourth ionization energy values are more rapid. However, the trends in these values show the usual discontinuity half way along the series. The reason is that the five d electrons are all unpaired, in singly occupied orbitals. When the sixth and subsequent electrons enter, the electrons have to share the already occupied orbitals resulting in inter-electron repulsions, which would require less energy to remove an electron. Hence, the third ionization energy curve for the last five elements is identical in shape to the curve for the first five elements, but displaced upwards by 580 kJ mol-1.
Electronic Configurations
The electronic configuration of the atoms of the first row transition elements are basically the same. It can be seen in the Table above that there is a gradual filling of the 3d orbitals across the series starting from scandium. This filling is, however, not regular, since at chromium and copper the population of 3d orbitals increase by the acquisition of an electron from the 4s shell. This illustrates an important generalization about orbital energies of the first row transition series. At chromium, both the 3d and 4s orbitals are occupied, but neither is completely filled in preference to the other. This suggests that the energies of the 3d and 4s orbitals are relatively close for atoms in this row.
In the case of copper, the 3d level is full, but only one electron occupies the 4s orbital. This suggests that in copper the 3d orbital energy is lower than the 4s orbital. Thus the 3d orbital energy has passed from higher to lower as we move across the period from potassium to zinc. However, the whole question of preference of an atom to adopt a particular electronic configuration is not determined by orbital energy alone. In chromium it can be shown that the 4s orbital energy is still below the 3d which suggests a configuration [Ar] 3d44s2. However due to the effect of electronic repulsion between the outer electrons the actual configuration becomes [Ar]3d54s1 where all the electrons in the outer orbitals are unpaired. It should be remembered that the factors that determine electronic configuration in this period are indeed delicately balanced.
| Redox Couple | E°/V |
| Mn2+(aq.)/Mn(s) | -1.18 |
| H+(aq.)/H2(g) | 0.00 |
This shows that elemental Mn is a stronger reductant than molecular hydrogen and hence should be able to displace hydrogen gas from 1 mol dm-3 hydrochloric acid.
Contributors and Attributions
Prof. Robert J. Lancashire (The Department of Chemistry, University of the West Indies)

