Reactions of Group 2 Elements with Oxygen
- Page ID
- 3677
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Group 2 elements (beryllium, magnesium, calcium, strontium and barium) react oxygen. to generate metal oxides. This Module addressed why it is difficult to observe a tidy pattern of this reactivity.
Formation of simple oxides
On the whole, the metals burn in oxygen to form a simple metal oxide. Beryllium is reluctant to burn unless it is in the form of dust or powder. Beryllium has a very strong (but very thin) layer of beryllium oxide on its surface, and this prevents any new oxygen getting at the underlying beryllium to react with it.
\[ 2X_{(s)} + O_{2(g)} \rightarrow 2XO_{(s)} \nonumber \]
with \(X\) representing any group 2 metal.
It is almost impossible to find any trend in the way the metals react with oxygen. It would be quite untrue to say that they burn more vigorously as you go down the Group. To be able to make any sensible comparison, you would have to have pieces of metal which were all equally free of oxide coating, with exactly the same surface area and shape, exactly the same flow of oxygen around them, and heated to exactly the same extent to get them started.
What the metals look like when they burn is a bit problematical!
- Beryllium: My best guess would be the same sort of silvery sparkles that magnesium or aluminum powder burn with if they are scattered into a flame - but I don't know that for sure.
- Magnesium burns with a typical intense white flame.
- Calcium is quite reluctant to start burning, but then bursts dramatically into flame, burning with an intense white flame with a tinge of red at the end.
- Strontium is also reluctant to start burning, but then burns with an intense almost white flame with red tinges especially around the outside.
- Barium: A pale green flame that appears to be white with some pale green tinges. It was not noticeably any more dramatic than the familiar magnesium flame.
Formation of Peroxides
Strontium and barium will also react with oxygen to form strontium or barium peroxide. Strontium forms this if it is heated in oxygen under high pressures, but barium forms barium peroxide just on normal heating in oxygen. Mixtures of barium oxide and barium peroxide will be produced.
\[ Ba_{(s)} + O_{2(s)} \rightarrow BaO_{2(s)} \nonumber \]
The strontium equation would look just the same.
The Reactions with Air
The reactions of the Group 2 metals with air rather than oxygen is complicated by the fact that they all react with nitrogen to produce nitrides. In each case, you will get a mixture of the metal oxide and the metal nitride. The general equation for the Group is:
\[ 3X_{(s)} + N_{2(g)} \rightarrow X_3N_{2(s)} \nonumber \]
For example, the familiar white ash you get when you burn magnesium ribbon in air is a mixture of magnesium oxide and magnesium nitride.
\[ 2Mg_{(s)} + O_{2(g)} \rightarrow 2MgO_{(s)} \nonumber \]
\[ 3Mg_{(s)} + N_{2(g)} \rightarrow Mg_3N_{2(s)} \nonumber \]
There are no simple patterns in the way the metals burn. While it would be tempting to say that the reactions get more vigorous as you go down the Group, but it is not true. The overall amount of heat evolved when one mole of oxide is produced from the metal and oxygen also shows no simple pattern:
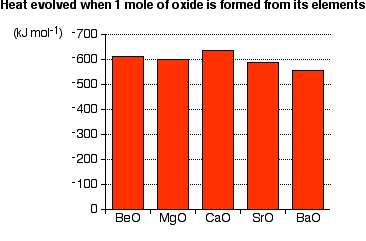
If anything, there is a slight tendency for the amount of heat evolved to decrease as you go down the Group. But how reactive a metal seems to be depends on how fast the reaction happens (i.e., Kinetics) - not the overall amount of heat evolved (i.e., Thermodynamics). The speed is controlled by factors like the presence of surface coatings on the metal and the size of the activation energy.
You could argue that the activation energy will fall as you go down the Group and that will make the reaction go faster. The activation energy will fall because the ionization energies of the metals fall. In this case, though, the effect of the fall in the activation energy is masked by other factors - for example, the presence of existing oxide layers on the metals, and the impossibility of controlling precisely how much heat you are supplying to the metal in order to get it to start burning.
Why do some metals form peroxides on heating in oxygen?
Beryllium, magnesium and calcium don't form peroxides when heated in oxygen, but strontium and barium do. There is an increase in the tendency to form the peroxide as you go down the Group. The peroxide ion, O22- looks like this:
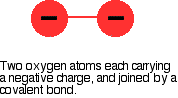
The covalent bond between the two oxygen atoms is relatively weak. Now imagine bringing a small 2+ ion close to the peroxide ion. Electrons in the peroxide ion will be strongly attracted towards the positive ion. This is then well on the way to forming a simple oxide ion if the right-hand oxygen atom (as drawn below) breaks off.
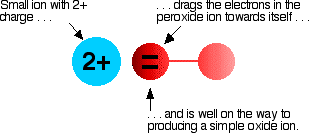
We say that the positive ion polarizes the negative ion. This works best if the positive ion is small and highly charged - if it has a high charge density.
A high charge density simply means that you have a lot of charge packed into a small volume.
Ions of the metals at the top of the Group have such a high charge density (because they are so small) that any peroxide ion near them falls to pieces to give an oxide and oxygen. As you go down the Group and the positive ions get bigger, they don't have so much effect on the peroxide ion. For example, Barium peroxide can form because the barium ion is so large that it doesn't have such a devastating effect on the peroxide ions as the metals further up the Group.
Why do these metals form nitrides on heating in air?
Nitrogen is often thought of as being fairly unreactive, and yet all these metals combine with it to produce nitrides, X3N2, containing X2+ and N3- ions. Nitrogen is fairly unreactive because of the very large amount of energy is required to break the triple bond joining the two atoms in the nitrogen molecule, N2. When something like magnesium nitride forms, you have to supply all the energy needed to form the magnesium ions as well as breaking the nitrogen-nitrogen bonds and then forming N3- ions. All of these processes absorb energy. This energy has to be recovered from somewhere to give an overall exothermic reaction - if the energy can't be recovered, the overall change will be endothermic and will not happen.
Energy is evolved when the ions come together to produce the crystal lattice (lattice energy or enthalpy). The size of the lattice energy depends on the attractions between the ions. The lattice energy is greatest if the ions are small and highly charged - the ions will be close together with very strong attractions. In the whole of Group 2, the attractions between the 2+ metal ions and the 3- nitride ions are big enough to produce very high lattice energies. When the crystal lattices form, so much energy is released that it more than compensates for the energy needed to produce the various ions in the first place. The excess energy evolved makes the overall process exothermic.
This is in contrast to what happens in Group 1 of the Periodic Table (lithium, sodium, potassium, rubidium and cesium). Their ions only carry one positive charge, and so the lattice energies of their nitrides will be much less.
Lithium is the only metal in Group 1 to form a nitride. Lithium has by far the smallest ion in the Group, and so lithium nitride has the largest lattice energy of any possible Group 1 nitride. Only in lithium's case is enough energy released to compensate for the energy needed to ionize the metal and the nitrogen - and so produce an exothermic reaction overall. In all the other Group 1 elements, the overall reaction would be endothermic. Those reactions don't happen, and the nitrides of sodium and the rest are not formed.
Contributors and Attributions
Jim Clark (Chemguide.co.uk)


