Buchwald-Hartwig Amination
- Page ID
- 69086
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
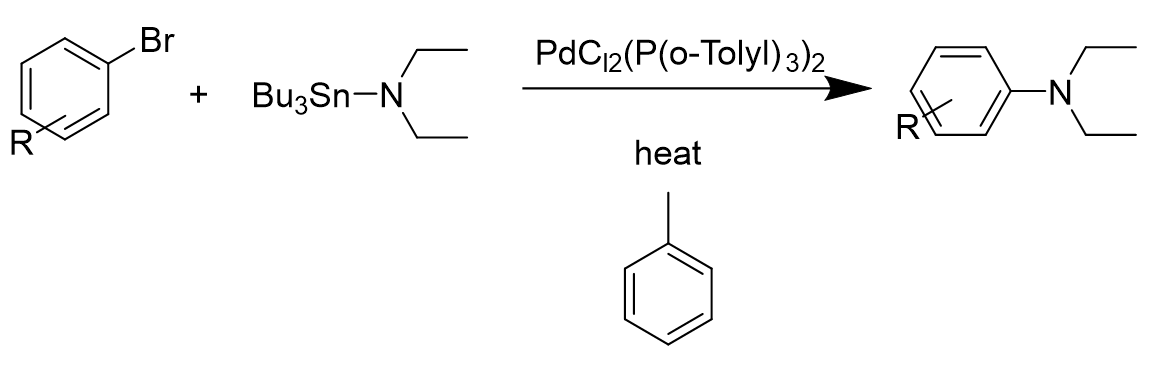
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Buchwald-Hartwig amination is a palladium-catalyzed cross-coupling reaction of amines and aryl halides that results in formation of C-N bonds. It was first introduced by Kosugi, Kameyama and Migita in 1983[1]. It was a reaction using 1 mol% PdCl2(P(o-Tolyl3)2 with the addition of aryl bromides and N,N-diethylamino-tributyltin in toluene solvent heated at 100°C for three hours. The resulting data showed that only nonsubstitued bromobenzene would give the product with a high yield.
In the following year, Pd(PPh3)4 catalyst mediated -carboline preparation was used to synthesize lavendamycin CDE ring system from 4-aryl pyridines, which was done by Bogen and Panek[2]. After a decade, Hartwig[3] identified and characterized several intermediates in the palladium-catalyzed C-N bond formation. The mechanistic data suggested that the reaction involved oxidation addition and reductive elimination steps.
In the same year, Buchwald[4] published a paper that discussed methods of improving original studies from Migita. One substrate was a volatile amine and the other was an amine with higher boiling point, and the reaction of these two substrates would result in the formation of aminostannes. The transamination was coupled with palladium catalyst to make the reactions available to a broader variety of arylamine substrates.
Mechanism
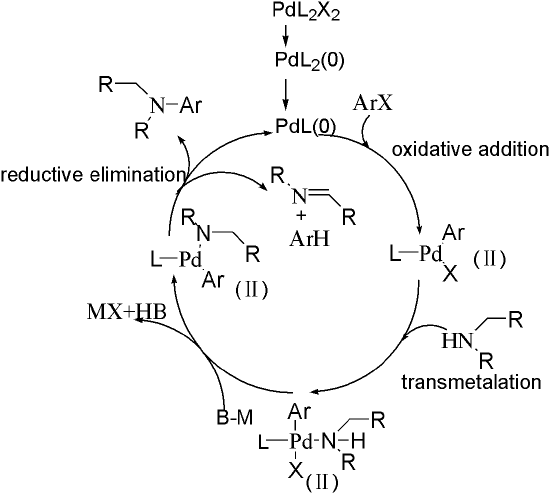
The catalysis circle is shown in Figure 1. First, Pd(Ⅱ) is reduced to Pd(0) by amines that contain α-H or ligand. Then, Pd kicks one ligand off and undergoes oxidative addition to form Pd(Ⅱ) complex. Next, amines attack Pd, substituting one X under the help of base. The final step is reductive elimination, giving the product and complete the circle. Note that reduction of Pd(Ⅱ) requires amines that contain α-H, otherwise extra ligands should be added to the reaction. An alternative choice is using Pd(0) complex instead of Pd(OAc)2.
Ligands
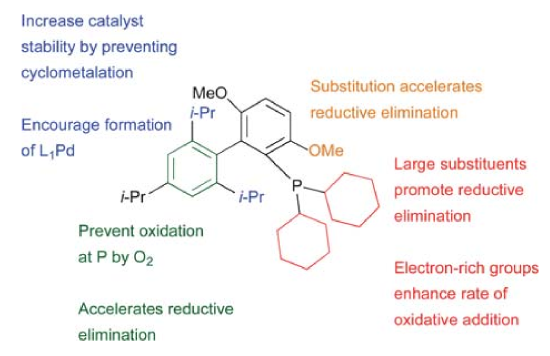
Buchwald proposed general ligand design strategy shown below. They changed the functional groups to get a library of ligands, for different substrates. Different ligands will be discussed in detail in Scope part. [5]
Electrophile
RX and ArX
Generally, Br, Cl and I can react with amines in certain conditions. ArI is relatively difficult, unlike other C-C coupling reactions. Mechanism studies showed that this results from the unreactive Pd dimers bridged by iodide anions.[5]
Scheme 1
Toluene is favored for this reaction because of poor solubility of Iodine salt in toluene.
ArOTf, ArONf and ArOMs
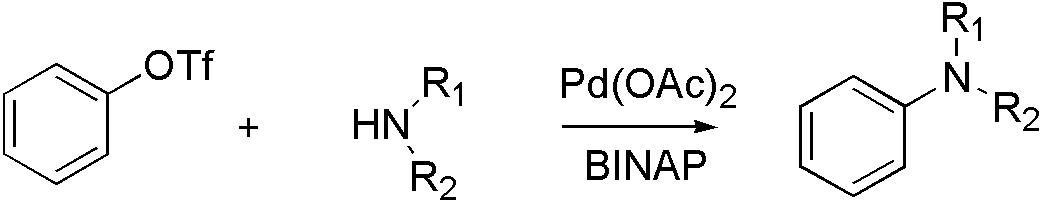
ArOTf[6]
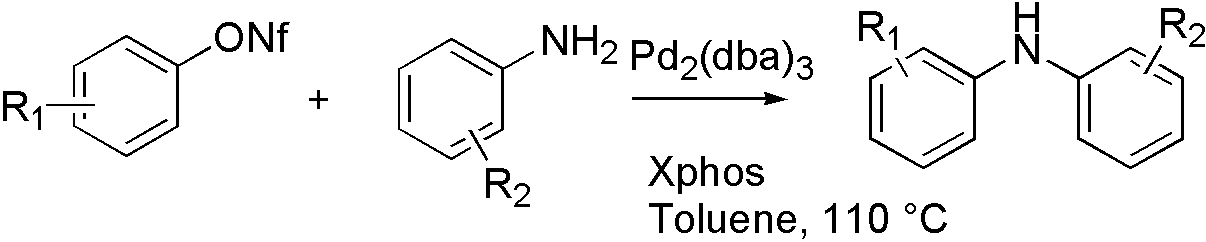
ArONf[7]
ArOMs[8]
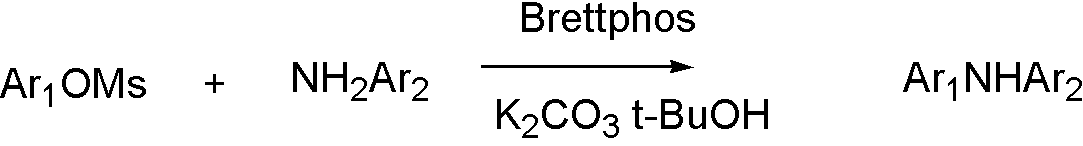
Scheme 2
OTf, ONf, and OMs broaden the range that Buchwald coupling applies, which means that hydroxyl group can react with amine by using OTf.
Nucleophiles
Primary amines
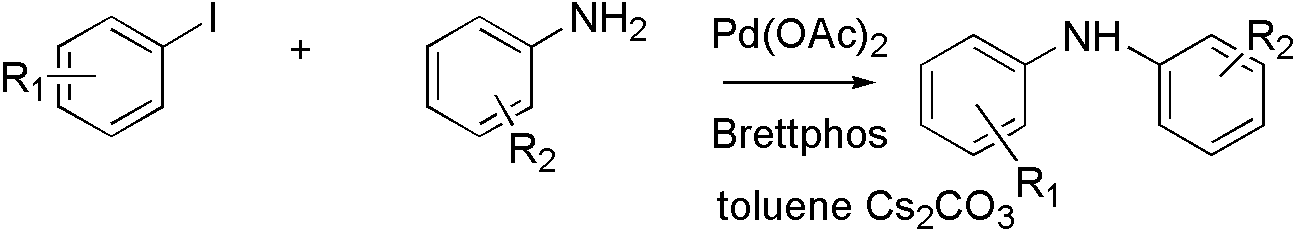
Brettphos[9] is the ligand designed for primary amines.
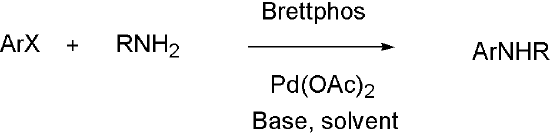
Scheme 3
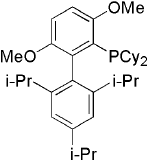
Brettphos has selectivity in primary amines toward secondary amines. Using LiHMDS as base combined with Brettphos can get proton tolerance like hydroxyl and carboxyl.
Secondary amines
Everything is almost the same with primary amines, instead of ligand. Ruphos[9] is designed for secondary amines.
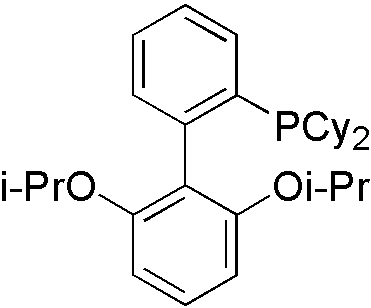
Similarly, LiHMDS is utilized to gain proton tolerance. However, it is not easy to have selectivity in secondary amines toward primary amines by steric hindrance control.
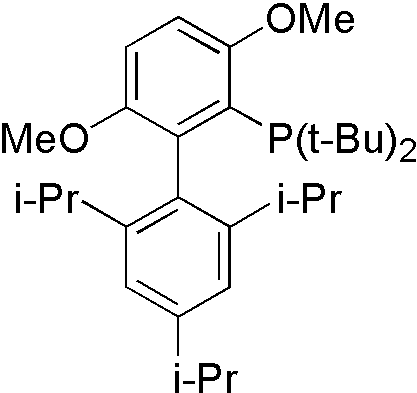
Amide[10]
Scheme 4:
Amide is not a good nucleophile, so more reactive ligand tBuBrettpos[10] is designed to solve this problem.
- NH heterocyclic compounds[11]
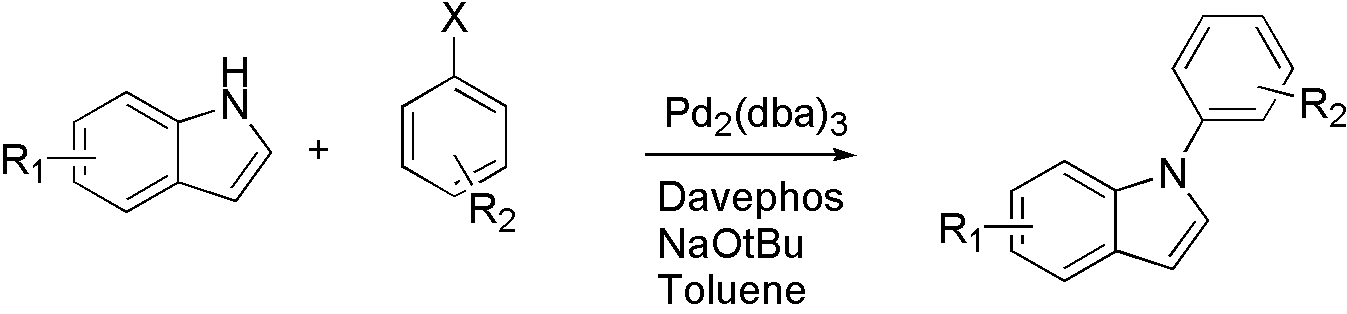
Scheme 5
For different types of nucleophile, different ligand is used. For indole, Davephos[11] is a good choice.
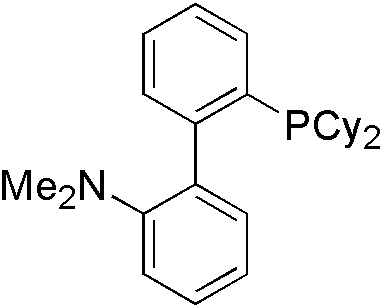

Similarly, tBuXphos[12] is for indazole[13].
Scope
It is critical to choose the correct coordinating ligands to the palladium in the Buchwald-Hartwig amination. For intramolecular coupling reaction of aryl bromides with amines having stereocenters at the α-position to the nitrogen atom, the use of Pd(P(o-tolyl)3) would not give racemic mixtures of products. Instead, it would form products with high enantiopurity. [14]
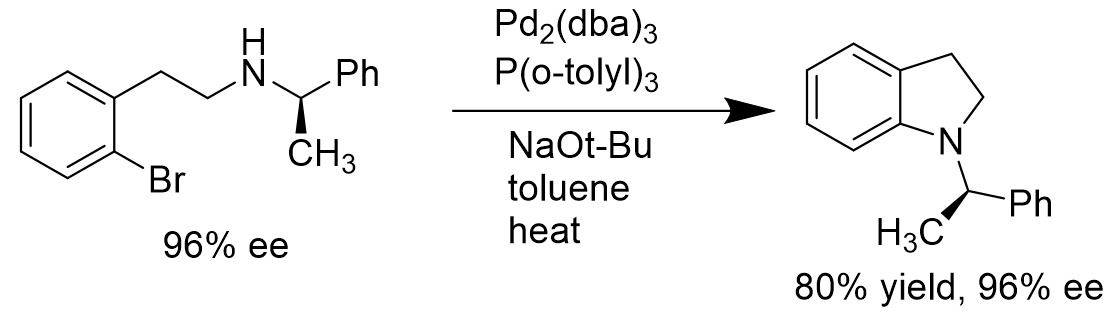
Scheme 6
However, in the case of intermolecular coupling reactions, it is catalyzed by Pd(BINAP) to give the coupled products without loss of enantioselectivity. [14]
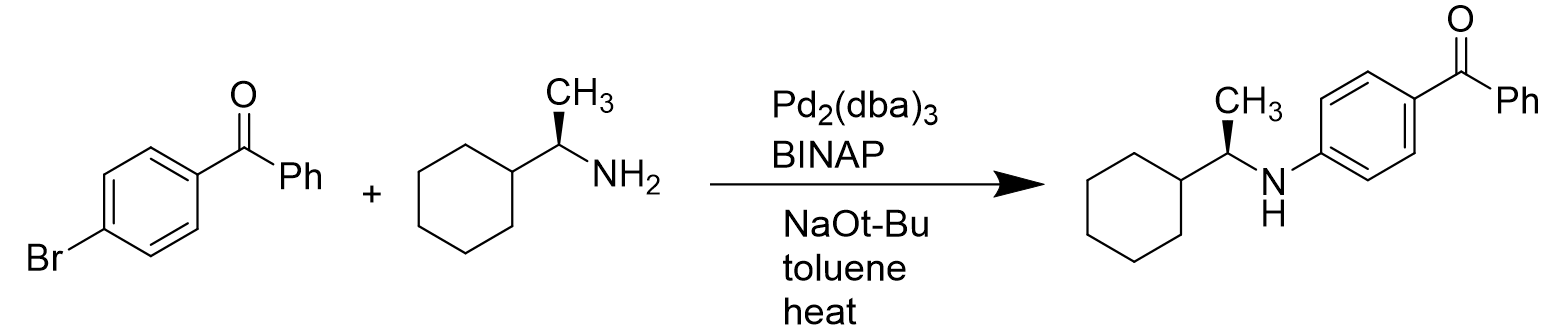
Scheme 7
Choice of base and catalysts
For base and catalysts choice, Buchwald gave a good summary in his “user guide”. We cite it here.[5]
| Base | Advantages | Disadvantages |
|---|---|---|
| NaOT-Bu | Permits highest reaction rates and lowest catalysis loadings | Incompatible with many electrophilic functional groups |
| LHMDS |
Allows utilization of substrates bearing protic functional groups Useful for low temperate amination |
Solid base is air sensitive Incompatible with some functional groups at elevated temperature |
| \(Cs_2CO_3\) | Provides excellent functional group tolerance and often highest reaction rate of weak base |
Expensive can be hard to stir on large scale |
| \(K_3PO_4\) and \(K_3CO_4\) |
Excellent functional group tolerance Often most efficient for arylation of amides Economically attractive |
Can require relatively high catalyst loadings and long reactions times |

Advantages and Limitations
Buchwald reactions have many advantages.[5] The catalyst loading is relative low, around 1%-2%, and all ligands are commercially available. It can be done in THF, toluene, t-BuOH and dioxane, and little amount of water is fine. Sometimes water is added intentionally to help Pd(Ⅱ) reduction. Reaction requires argon protected environment, but the reaction system is not very sensitive to oxygen.
As to scope, Buchwald-Hartwig reaction can be applied to various amines, which is discussed above, and most of them have a very good yield. Proton tolerance can be acquired by using LiHMDS. However, functional groups like azo may cause catalyst poisoning, messing the reaction up. Esters and nitro groups are incompatible with KOtBu, but weak base like K2CO3 has a low reaction rate. For more details of this reaction, just turn to Buchwald’s “user guide” published in Chem. Sci. Buchwald-Hartwig reaction is of great significance, which provides a strong tool for C-N coupling.
Reference
- M. Kosugi, M. Kameyama and T. Migita, Chemistry Letters, 1983, 12, 6, 927-928.
- D. Bogen and J. Panek, Tetrahedron Lett., 1984, 25, 30, 3175-3178.
- F. Paul, J. Patt and J. F. Hartwig, J. Am. Chem. Soc., 1994, 116, 5969-5970.
- A. S. Guram and S. L. Buchwald, J. Am. Chem. Soc., 1994, 116, 17, 7901-7902.
- D.S.Surry and S.L.Buchwald, Chem.Sci., 2011, 2, 27, 27-50.
- J. Ahman and S. L. Buchwald, Tetrahedron Lett., 1997, 38, 6363-6366.
- R. E. Tundel, K. W. Anderson and S. L. Buchwald, J. Org. Chem., 2006, 71, 430-433.
- B. P. Fors, D. A. Watson, M. R. Biscoe and S. L. Buchwald, J. Am. Chem. Soc., 2008, 130, 41, 13552-13554.
- D. Maiti, B. P. Fors, J. L. Henderson, Y. Nakamura and S. L. Buchwald, Chem. Sci., 2011, 2, 57-68.
- B. P. Fors, K. Dooleweerdt, Q. Zeng and S. L. Buchwald, Tetrahedron Lett., 2009, 65, 6576-6583.
- D. W. Oldm M. C. Harris and S. L. Buchwald, Org. Lett., 2000, 2, 10, 1403-1406.
- X. Huang, K. W. Anderson, D. Zimi, L. Jiang, A. Klapers and S. L. Buchwald, J. Am. Chem. Soc., 2003, 125, 6653-6655.
- K. W. Anderson, R. E. Tundel, T. Ikawa, R. A. Altman and S. L. Buchwald, Angew. Chem. Int. Ed., 2006, 45, 6523-6527.
- S.Wagaw, R.A.Rennel, and S.L.Buchwald, J.Am.Chem.Soc., 1997, 119, 8451-8458.
Contributors and Attributions
- Shiyu Chen (New York University) and Shuhui Chen (New York University)

