17.7A: Interhalogen Compounds
- Page ID
- 36210
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Some selected properties of the hydrogen halides (HX) are given in the table below [1].
| Property of HX | HF | HCl | HBr | HI |
|---|---|---|---|---|
| Physical appearance at 298K | Colorless gas | Colorless gas | Colorless gas | Colorless gas |
| Melting point /K | 189 | 159 | 186 | 222 |
| Boiling point /K | 293 | 188 | 207 | 237.5 |
| Liquid Range /K | 104 | 29 | 21 | 15.5 |
| ΔfusH°(mp) / kJ mol-1 | 4.6 | 2 | 2.4 | 2.9 |
| ΔvapH°(bp) / kJ mol-1 | 34 | 16.2 | 18 | 19.8 |
| ΔfH°(298 K) / kJ mol-1 | -273.3 | -92.3 | -36.3 | +26.5 |
| ΔfG°(298 K) / kJ mol-1 | -275.4 | -95.3 | -53.4 | +1.7 |
| Bond dissociation energy / kJ mol-1 | 570 | 432 | 366 | 298 |
| Bond length /pm | 92 | 127.5 | 141.5 | 161 |
| Dipole moment /D | 1.83 | 1.11 | 0.83 | 0.45 |
| predicted pKa's | 1.4 | -9.3 | -11.7 | -12.4 |
At room temperature, all of the halogen halides are gases and have sharp, acid smells. They can be prepared by direct combination of the halogens with H2 or by the action of a concentrated acid (nonoxidising for HBr and HI) on metal halides.
\[CaF_{2(s)} + H_2SO_{4(l)} → CaSO_{4(s)} + 2HF_{(g)}\]
The reaction is exothermic so to optimise yields it is done at 473-523 °K for 30-60 minutes. Sulfuric acid is too strong an oxidising agent to be used in the generation of HBr and HI resulting in partial oxidation, for example:
\[2HBr + H_2SO_4 → Br_2+ 2H_2O + SO_2\]
Phosphoric acid can be used instead though:
\[3NaBr_{(s)} + H_3PO_4 → Na_3PO_{4(s)} + 3HBr_{(g)}\]
Aqueous solutions of HX are generally referred to as hydohalic acids and we will look at some chemistry of both the anhydrous hydrogen halides and the hydrohalic acids.
Commercial production of anhydrous Hydrogen Fluoride began in the 1930's and by the 1980's at least 16 countries were involved in generating over 1 million tonnes worldwide. Initially the HF was used in making refrigerants and for synthetic cryalite Na2AlF6 for aluminium production as well as in uranium processing. A small amount is used in glass etching. Hydrofluoric acid attacks glass by reaction with silicon dioxide to form gaseous or water soluble silicon fluorides. The dissolution process proceeds as follows:
\[SiO_2 + 4 HF → SiF_{4 (g)} + 2 H_2O\]
\[SiO_2 + 6 HF → H_2SiF_6 + 2 H_2O\]
This property has been known since the 17th century, even before a general procedure for the preparation of large quantities of hydrofluoric acid had been devised by Scheele in 1771. Small quantities of HF(aq) are shipped in TEFLON or polyethylene-lined containers with which it does not react.

Figure 1: Edged glass from the Bankfield Museum, Halifax, West Yorkshire, England. (53.43'.57".N; 1.51'.48".W.). from Wikipedia.
HF is miscible with water in all proportions and phase diagrams show several distinct species including
- HF.H2O (mp 237.7 °K)
- 2HF.H2O (mp 197.7 °K)
- 4HF.H2O (mp 172.8 °K).
An interesting high-tech application of HF/DF is in High-Energy Lasers as weapons which were first demonstrated in the 1970's and have now reached the level of being mobile and capable of shooting down incoming missiles. The construction of a deuterium fluoride laser resembles a rocket engine. In the combustion chamber, ethylene is burned in nitrogen trifluoride. This reaction produces free excited fluorine radicals. Just after the nozzle, the mixture of helium and hydrogen or deuterium gas is injected to the exhaust stream; the hydrogen or deuterium reacts with the fluorine radicals, producing excited molecules of deuterium or hydrogen fluoride. The excited molecules then undergo stimulated emission in the optical resonator region of the laser.
Deuterium fluoride lasers have found military applications: the MIRACL laser, the Pulsed Energy Projectile and the Tactical High Energy Lasers are of the deuterium fluoride type. The HF laser is somewhat cheaper and operates at 2.7-2.9 µm and this is affected by the atmosphere. The DF laser operates at 3.8 µm and so is considered more useful for terrestrial applications.
Hydrogen chloride
Hydrogen chloride is one of the the largest volume chemicals to be manufactured as either the gas or aqueous acid. Total world production is estimated at 20 Mt/year. When a cheap source of NaCl was available the two-stage Leblanc process, developed during the Industrial Revolution of the 1700's, was used:
\[NaCl + H_2SO_4 → NaHSO_4 + HCl\]
\[NaCl + NaHSO_4 → Na_2SO_4 + HCl\]
Here salt is converted to sodium sulfate, using sulfuric acid, giving hydrogen chloride as by-product. Initially, this gas was released to the air, but the Alkali Act of 1863 in the UK prohibited such release, the manufacturers then absorbed the HCl waste gas in water, producing hydrochloric acid on an industrial scale. Modern production of HCl is still as a byproduct that is recovered from large scale processes but now more likely to be from an Industrial Organic Scheme.
Commercial HCl is available up to about 40%, above which the evaporation rate makes it too unstable. Industrially it is usually sold at 30-35% in strength and its major use is for cleaning iron surfaces, where 18% is used. Pickling or removal of rust (iron oxide scale) from steel involves the following reaction:
\[Fe_2O-3 + Fe + 6 HCl → 3 FeCl_2 + 3 H_2O\]
The HCl is then regenerated via a closed loop reaction scheme that has been developed so that Fe2O3 is recovered as a by-product as well. When high-purity HCl is required, then burning of dihydrogen in dichlorine is used. This is a highly exothermic reaction. High quality HCl is used in the food and pharmaceutical industry as well as for water treatment.
The acid in our stomachs (gastric acid) consists mainly of HCl (around 0.5%), and large quantities of potassium chloride and sodium chloride. The pH is usually around 1-2.
The infrared spectrum of gaseous hydrogen chloride consists of a number of sharp absorption lines grouped around 2886 cm-1 and for deuterium chloride vapor this has shifted to 2090 cm-1. These classic molecular spectra can be analyzed to provide information about both rotational and vibrational energies of the molecules. The absorption lines shown involve transitions from the ground to the first excited vibrational state of HCl/DCL, but also involve changes in the rotational states. The rotational angular momentum changes by 1 during such transitions. The splitting of the lines shows the difference in rotational inertia of the two chlorine isotopes 35Cl (75.5%) and 37Cl (24.5%). Calculation of the bond length for H-35Cl for example gives a value of 131 pm, close to the accepted value of 127 pm.
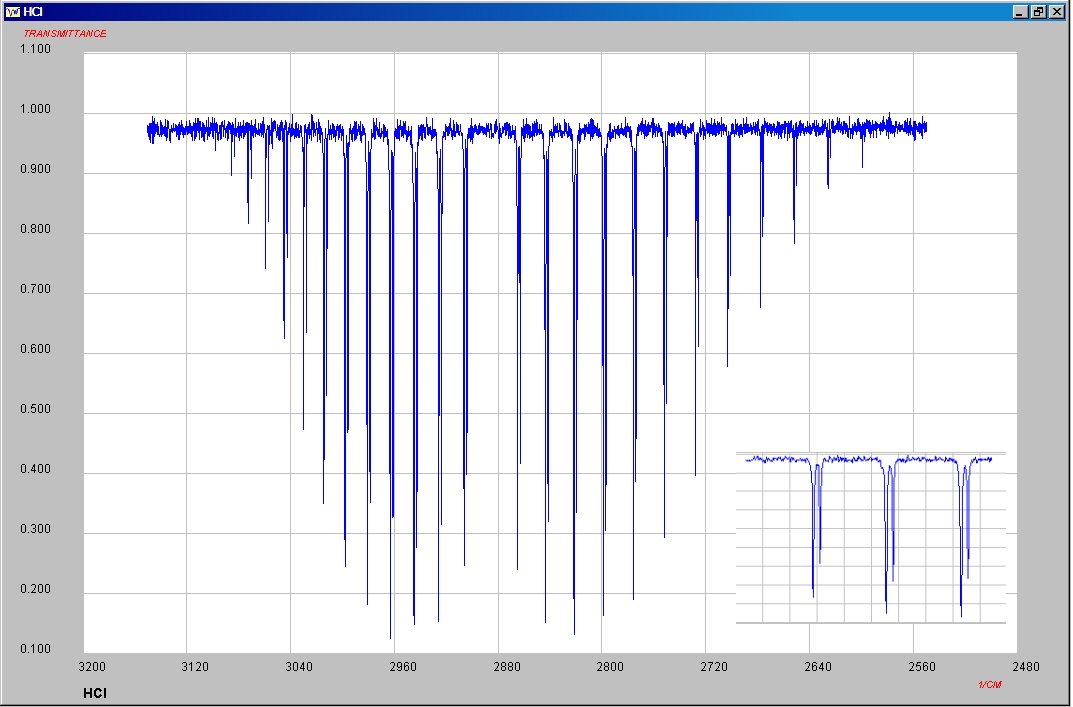
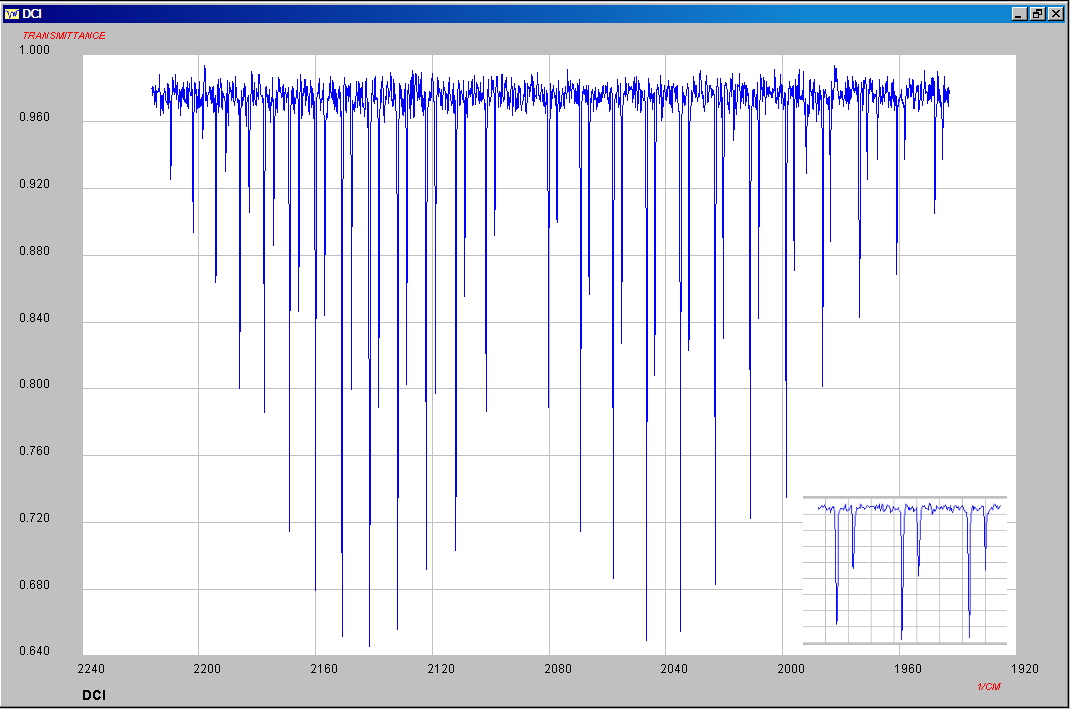
Both HBr and HI can be produced by direct combination, using platinum catalysts and elevated temperatures. An alternate method for the production of HI is the quantitative reaction of iodine with hydrazine.
\[2I_2 + N_2H_4 → 4HI + N_2\]
Hydrogen Bromide is very water soluble and aqueous HBr becomes saturated at around 69% by weight at room temperature. A constant boiling point mixture exists at about 47.6% and this boils at 124.3 °C. Boiling more dilute solutions will evaporate some of the water first to eventually arrive at a concentration of 47%.
Hydrogen iodide is extremely soluble in water (425 dm-3 in 1 dm-3, which is roughly equivalent to only 4 water molecules per HI molecule). The hydroiodic acid produced is the strongest acid in the series, pka ~-10.
HI(g) + H2O(l) → H3O+(aq) + I -(aq) pKa ~ -10
HBr(g) + H2O(l) → H3O+(aq) + Br-(aq) pKa ~ -9
HCl(g) + H2O(l) → H3O+(aq) + Cl-(aq) pKa ~ -8
Melting and Boiling Points
As with the dihalogens, it is expected that with increasing size and polarizability the MP's and BP's should increase. The position of HF though needs further explanation.
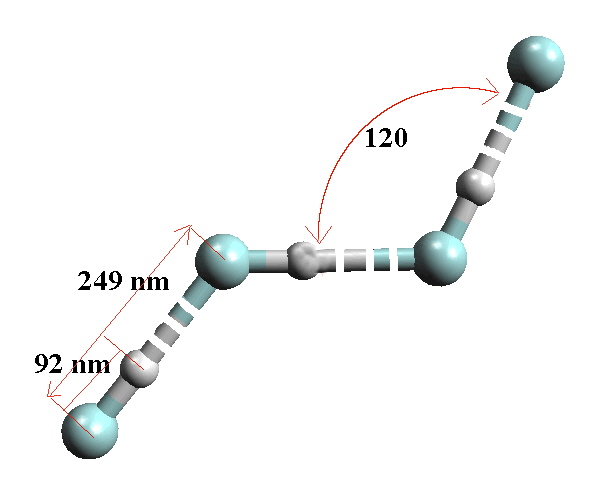
There is extensive H-bonding in HF in all states and this explains why HF has such a high boiling point (293 °K), even higher than the much heavier HI. Solid HF exists as an H-bonded polymer.
Acidic properties
In water, the hydrohalic acids are formed by reaction with the gaseous hydrogen halides and apart from HF these are completely dissociating strong acids. In the case of HF, where there is a short, strong bond, (bond dissociation energy of 570 kJ mol-1) only a weak acid is formed (pKa=3.45). A qualitative explanation for this behavior is related to the tendency of HF to hydrogen-bond and form ion-pair clusters such as [H3O+F-]
\[H_2O + HF → [H_3O^+F^-] → H_3O^+ + F^-\]
The first step is thought to lie well to the right, but the 2nd step perhaps only 15%. The reaction representing the pK is given by:
HX(aq) → H+(aq) + X-(aq)ΔG° = -RTln(K)
ΔG° = ΔH° - TΔS°
The factors that influence the degree of dissociation can not all be readily measured but can be simplified as the following 6 steps in the Hess cycle. [1]
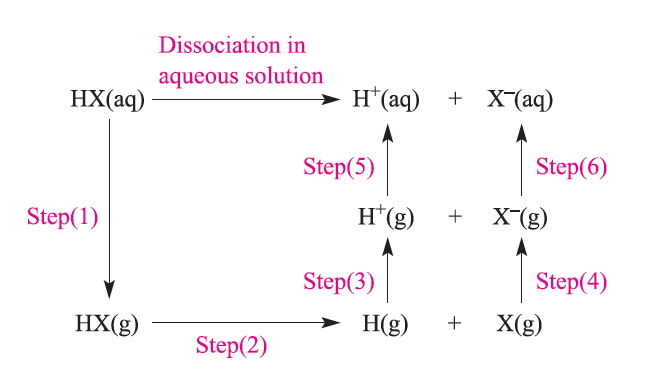
Step 1 must be predicted since it is based on having undissociated HX(aq), and apart from HF the hydrogen halides are completely dissociated in aqueous solution. Step 2 is the cleavage of the HX bond. Steps 3 and 5 are independent of the halide and are for the ionisation and hydration of the H+ ion. Step 4 is related to the Electron Affinity and step 6 is the hydration of the gaseous X- ion.
A plot of the energies of steps 1,2,4 and 6 shows little variation for the heavier halides so that it appears that considering only the ΔH terms is not sufficient to explain the trend and it is only when the TΔS terms are considered as well that the ΔG° for HF is predicted to be positive whereas the others are all negative. The predicted value for HF is 1.4 but experimentally the value is found to be 3.45, the trend is accurately predicted but there is clearly much room for improvement!
Safety
In contrast to the weak acidicity of dilute aqueous HF solutions, in concentrated hydrogen fluoride solution, F- ions form a [HF2]-(aq) complex by addition to HF molecules. HF molecules remain ionised to compensate the loss of F- ions. More H+ ions are thus formed, making concentrated HF an effectively strong acid. Anhydrous hydrogen fluoride is an extremely strong acid comparable in strength to anhydrous sulfuric acid.
The fact that it is a weak acid in dilute solutions does not mean it is any less of a hazard and the poorly dissociated HF actually penetrates tissue more quickly than typical acids. Symptoms of exposure to hydrofluoric acid may not be immediately evident and unfortunately any delay in treatment can lead to serious consequences. The severe pain that comes on is thought to be caused by dissolution of Ca in the bone to form insoluble CaF2 and it is has been found necessary in some cases to amputate affected limbs.
Interhalogens
Some properties of interhalogen compounds are listed below. They are all prepared by direct combination of the elements although since in some cases more than one product is possible the conditions may vary by altering the temperature and relative proportions. For example under the same conditions difluorine reacts with dichlorine to give ClF with dibromine to give BrF3 but with diiodine to give IF5.[1]
| Compound | ClF | BrF | BrCl | ICl | IBr | ClF3 | BrF3 | IF3 | I2Cl6 | ClF5 | BrF5 | IF5 | IF7 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Appearance at 298K | Colorless gas | Pale brown gas | impure | Red solid | Black solid | Colorless gas | Yellow liquid | Yellow solid | Orange solid | Colorless gas | Colorless liquid | Colorless liquid | Colorless gas |
| Stereochemistry | linear | linear | linear | linear | linear | T-shaped | T-shaped | T-shaped | planar | square-based pyramid | square-based pyramid | square-based pyramid | pentagonal bipyramid |
| Melting point /K | 117 | ~240 | dissoc. | 300(a) | 313 | 197 | 282 | 245 (dec) | 337 (sub) | 170 | 212.5 | 282.5 | 278 (sub) |
| Boiling point /K | 173 | ~293 | ~278 | ~373 | 389 | 285 | 399 | - | - | 260 | 314 | 373 | - |
| ΔfH°(298 K) /kJ mol-1 | -50.3 | -58.5 | 14.6 | -23.8 | -10.5 | -163.2 | -300.8 | ~-500 | -89.3 | -255 | -458.6 | -864.8 | -962 |
| Dipole moment for gas-phase molecule /D | 0.89 | 1.42 | 0.52 | 1.24 | 0.73 | 0.6 | 1.19 | - | 0 | - | 1.51 | 2.18 | 0 |
| Bond distances in gas-phase molecules except for IF3 and I2Cl6 / pm | 163 | 176 | 214 | 232 | 248.5 | 160 (eq), 170 (ax) | 172 (eq), 181 (ax) | 187 (eq), 198 (ax) | 238 (terminal) 268 (bridge) | 172 (basal), 162 (apical) | 178 (basal), 168 (apical) | 187 (basal), 185 (apical) | 186 (eq), 179 (ax) |
Valence-shell electron-pair repulsion theory, VSEPR
The premise of the VSEPR is that the valence electron pairs surrounding an atom mutually repel each other, and will therefore adopt an arrangement that minimizes this repulsion, thus determining the molecular geometry. The number of electron pairs surrounding an atom, both bonding and nonbonding, is called its steric number. The VSEPR theory thus provides a simple model for predicting the shapes of such species, in particular for main group compounds. The model combines original ideas of Sidgwick and Powell (1940's) with extensions developed by Nyholm and Gillespie (1950's).
- Each valence shell electron pair of the central atom E in a molecule EXn containing E-X single bonds is stereochemically significant, and repulsions between them determine the molecular shape.
- Electron-electron repulsions decrease in the sequence: lone pair-lone pair > lone pair-bonding pair > bonding pair-bonding pair.
- Where the central atom E is involved in multiple bond formation to atoms X, electron-electron repulsions decrease in the order: triple bond-single bond > double bond-single bond > single bond-single bond.
- Repulsions between the bonding pairs in EXn depend on the difference between the electronegativities of E and X; electron-electron repulsions are less the more the E.X bonding electron density is drawn away from the central atom E.
The VSEPR theory works best for simple halides of the p-block elements, but may also be applied to species with other substituents. However, the model does not take steric factors (i.e. the relative sizes of substituents) into account.+
| Steric No. | Basic Geometry 0 lone pair |
1 lone pair | 2 lone pairs | 3 lone pairs |
|---|---|---|---|---|
| 2 | linear | |||
| 3 | trigonal planar | bent | ||
| 4 | tetrahedral | trigonal pyramid | bent | |
| 5 | trigonal bipyramid | seesaw | T-shaped | linear |
| 6 | octahedral | square pyramid | square planar | |
| 7 | pentagonal bipyramid | pentagonl pyramid |
Structure of the Interhalogens
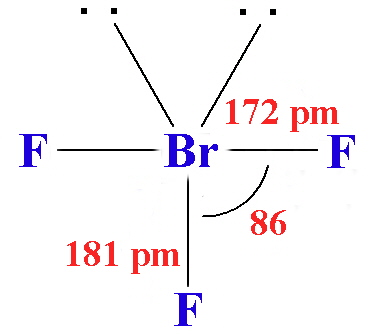
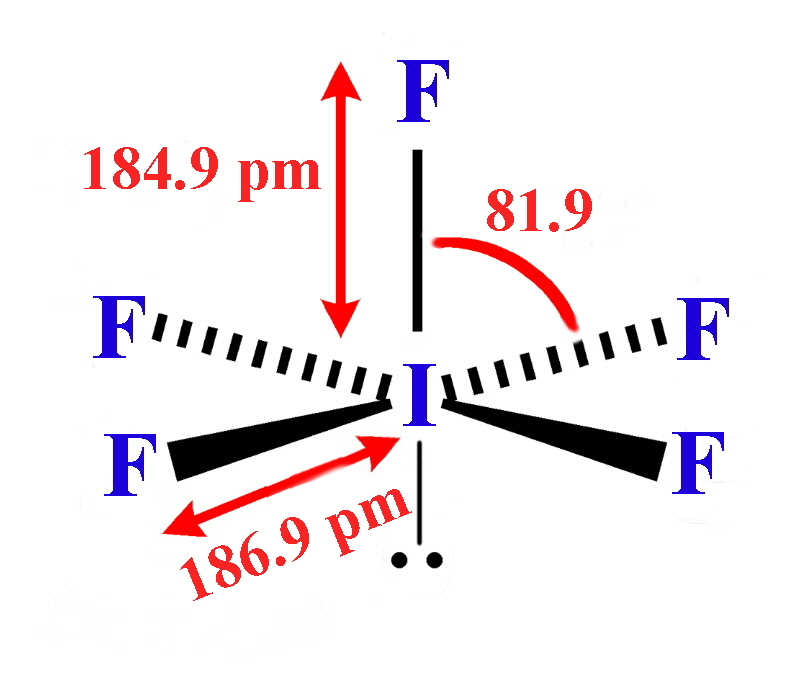
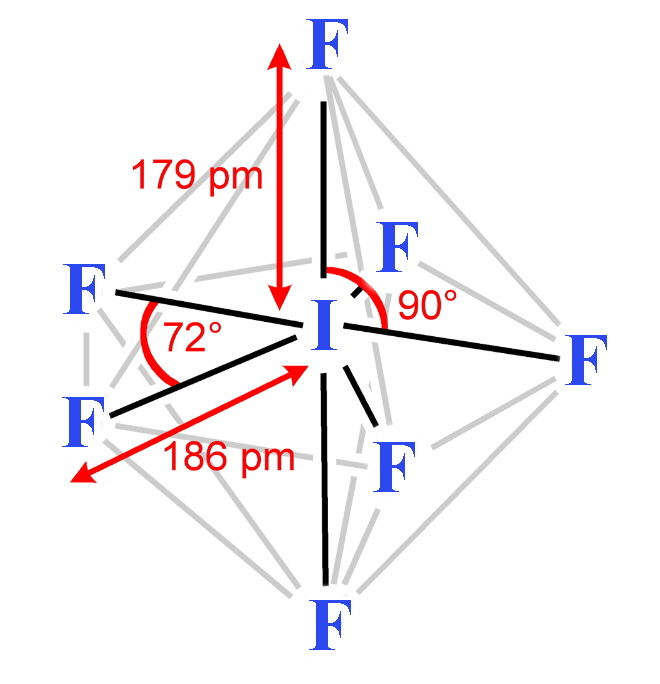
The structures found for the various interhalogens conform to what would be expected based on the VSEPR model. For XY3 the shape can be described as T-shaped with 2 lone pairs sitting in equatorial positions of a trigonal bipyramid. For XY5 the shape is a square pyramid with the unpaired electrons sitting in an axial position of an octahedral and XY7 is a pentagonal bipyramid.
XY diatomic interhalogens
The interhalogens with formula XY have physical properties intermediate between those of the two parent halogens. The covalent bond between the two atoms has some ionic character, the larger element, X, becoming oxidised and having a partial positive charge. Most combinations of F, Cl, Br and I are known, but not all are stable.
- Chlorine monofluoride (ClF), the lightest interhalogen, is a colorless gas with a boiling point of 173 °K.
- Bromine monofluoride (BrF) has not been obtained pure - it dissociates into the trifluoride and free bromine. Similarly, iodine monofluoride is unstable - iodine reacts with fluorine to form a pentafluoride.
- Iodine monofluoride (IF) is unstable and disproportionates rapidly and irreversibly at room temperature: 5IF → 2I2 + IF5. However, its molecular properties have been determined by spectroscopy: the iodine-fluorine distance is 190.9 pm and the I-F bond dissociation energy is around 277 kJ mol-1. ΔHf° = -95.4 kJ mol-1 and ΔGf° = -117.6 kJ mol-1, both at 298 K.
IF can be generated, by the following reactions:
I2 + F2 → 2IF at -45 °C in CCl3F;
I2 + IF3 → 3IF at -78 °C in CCl3F;
I2 + AgF → IF + AgI at 0 °C. - Bromine monochloride (BrCl) is an unstable red-brown gas with a boiling point of 5 °C.
- Iodine monochloride (ICl) consists of red transparent crystals which melt at 27.2 °C to form a choking brownish liquid (similar in appearance and weight to bromine). It reacts with HCl to form the strong acid HICl2. The crystal structure of iodine monochloride consists of puckered zig-zag chains, with strong interactions between the chains.
- Iodine monobromide (IBr) is made by direct combination of the elements to form a dark red crystalline solid. It melts at 42 °C and boils at 116 °C to form a partially dissociated vapor.
XY3 interhalogens
- Chlorine trifluoride (ClF3) is a Colorless gas that condenses to a green liquid, and freezes to a white solid. It is made by reacting chlorine with an excess of fluorine at 250° C in a nickel tube. It reacts more violently than fluorine, often explosively. The molecule is planar and T-shaped.
- Bromine trifluoride (BrF3) is a yellow green liquid that conducts electricity - it ionises to form [BrF2+] + [BrF4-]. It reacts with many metals and metal oxides to form similar ionised entities; with some others it forms the metal fluoride plus free bromine and oxygen. It is used in organic chemistry as a fluorinating agent. It has the same molecular shape as chlorine trifluoride.
- Iodine trifluoride (IF3) is a yellow solid which decomposes above -28 °C. It can be synthesised from the elements, but care must be taken to avoid the formation of IF5. F2 attacks I2 to yield IF3 at -45 °C in CCl3F. Alternatively, at low temperatures, the fluorination reaction I2 + 3XeF2 → 2IF3 + 3Xe can be used. Not much is known about iodine trifluoride as it is so unstable.
- Iodine trichloride (ICl3) forms lemon yellow crystals which can be melted under pressure to a brown liquid. It can be made from the elements at low temperature, or from iodine pentoxide and hydrogen chloride. It reacts with many metal chlorides to form tetrachloriodides, and hydrolyses in water. The molecule is a planar dimer, with each iodine atom surrounded by four chlorine atoms. In the melt it is conductive, which may indicate dissociation: \[I_2Cl_6 → ICl_2^+ + ICl_4^-\]
Chlorine trifluoride, ClF3 was first reported in 1931 and it is primarily used for the manufacture of uranium hexafluoride, UF6 as part of nuclear fuel processing and reprocessing, by the reaction:
\[U + 3 ClF_3 → UF_6 + 3 ClF\]
U isotope separation is difficult because the two isotopes have very nearly identical chemical properties, and can only be separated gradually using small mass differences. (235U is only 1.26% lighter than 238U.) A cascade of identical stages produces successively higher concentrations of 235U. Each stage passes a slightly more concentrated product to the next stage and returns a slightly less concentrated residue to the previous stage.
There are currently two generic commercial methods employed internationally for enrichment: gaseous diffusion (referred to as first generation) and gas centrifuge (second generation) which consumes only 6% as much energy as gaseous diffusion. These both make use of the volatility of UF6. ClF3 has been investigated as a high-performance storable oxidizer in rocket propellant systems. Handling concerns, however, prevented its use.
Hypergolic means explode on contact with no need for any activator. One observer made the following comment about \(ClF_3\):
It is, of course, extremely toxic, but that's the least of the problem. It is hypergolic* with every known fuel, and so rapidly hypergolic that no ignition delay has ever been measured. It is also hypergolic with such things as cloth, wood, and test engineers, not to mention asbestos, sand, and water � with which it reacts explosively. It can be kept in some of the ordinary structural metals-steel, copper, aluminium, etc.-because of the formation of a thin film of insoluble metal fluoride which protects the bulk of the metal, just as the invisible coat of oxide on aluminium keeps it from burning up in the atmosphere. If, however, this coat is melted or scrubbed off, and has no chance to reform, the operator is confronted with the problem of coping with a metal-fluorine fire. For dealing with this situation, I have always recommended a good pair of running shoes."[3]
It is believed that prior to and during World War II, ClF3 code named N-stoff ("substance N") was being stockpiled in Germany for use as a potential incendiary weapon and poison gas. The plant was captured by the Russians in 1944, but there is no evidence that the gas was actually ever used during the war.
XY5 interhalogens
- Chlorine pentafluoride (ClF5) is a Colorless gas, made by reacting chlorine trifluoride with fluorine at high temperatures and high pressures. It reacts violently with water and most metals and nonmetals.
- Bromine pentafluoride (BrF5) is a Colorless fuming liquid, made by reacting bromine trifluoride with fluorine at 200° C. It is physically stable, but reacts violently with water and most metals and nonmetals.
- Iodine pentafluoride (IF5) is a Colorless liquid, made by reacting iodine pentoxide with fluorine, or iodine with silver fluoride. It is highly reactive, even slowly with glass. It reacts with elements, oxides and carbon halides. The molecule has the form of a tetragonal pyramid.
- Primary amines react with iodine pentafluoride to form nitriles after hydrolysis with water. \[R-CH_2-NH_2 → R-CN\]
XY7 interhalogens [3]
- Iodine heptafluoride (IF7) is a Colorless gas. It is made by reacting the pentafluoride with fluorine. IF7 is chemically inert, having no lone pair of electrons in the valency shell; in this it resembles sulfur hexafluoride. The molecule is a pentagonal bipyramid. This compound is the only interhalogen compound possible where the larger atom is carrying seven of the smaller atoms.
- All attempts to form bromine heptafluoride have met with failure; instead, bromine pentafluoride and fluorine gas are produced.
References
- "Inorganic Chemistry" - C. Housecroft and A.G. Sharpe, Prentice Hall, 3rd Ed., Dec 2007, ISBN13: 978-0131755536, ISBN10: 0131755536, Chapter 17.
- "Chemistry of the Elements", Greenwood and Earnshaw,Elsevier.
Contributors and Attributions
Prof. Robert J. Lancashire (The Department of Chemistry, University of the West Indies)

