16.5B: Hydrogen Peroxide, \(H_2O_2\)
- Page ID
- 34271
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Hydrogen peroxide (H2O2) is the simplest peroxide (a compound with an oxygen-oxygen single bond) and in its pure form is a colourless liquid that is slightly more viscous than water. It is a strong oxidizer and is used as a bleaching agent and disinfectant. For safety reasons it is normally used as an aqueous solution, also colourless. For consumers, it is usually available from pharmacies at 3 and 6 wt% concentrations. The concentrations are sometimes described in terms of the volume of oxygen gas generated; one milliliter of a 20-volume solution generates twenty milliliters of oxygen gas when completely decomposed. For laboratory use, 100 volume, 30 wt% solutions are the most common. Concentrated H2O2, or 'high-test peroxide' is a reactive oxygen species and has been used as a propellant in rocketry. Diluted H2O2 (between 1.9% and 12%) mixed with ammonium hydroxide has been used to bleach human hair. The chemical's bleaching property lends its name to the phrase "Hollywood peroxide blonde". Hydrogen peroxide can be used for tooth whitening and when mixed with baking soda and salt forms a recipe for home-made toothpaste.
Discovery
Hydrogen peroxide was first described in 1818 by Louis Jacques Thénard, who produced it by treating barium peroxide with nitric acid. An improved version of this process used hydrochloric acid, followed by addition of sulfuric acid to precipitate the barium sulfate byproduct. Thénard's process was used from the end of the 19th century until the middle of the 20th century.
Pure hydrogen peroxide was long believed to be unstable as early attempts to separate it from the water, which is present during synthesis, all failed. This instability was due to traces of impurities (transition metals salts) which catalyze the decomposition of the hydrogen peroxide. Pure hydrogen peroxide was first obtained in 1894 - almost 80 years after its discovery - by Richard Wolffenstein, who produced it via vacuum distillation.
Determination of the molecular structure of hydrogen peroxide proved to be very difficult. In 1892 the Italian physical chemist Giacomo Carrara (1864-1925) determined its molecular weight by freezing point depression, which confirmed that its molecular formula was H2O2. At least half a dozen hypothetical molecular structures seemed to be consistent with the available evidence. In 1934, the English mathematical physicist William Penney and the Scottish physicist Gordon Sutherland proposed a molecular structure for hydrogen peroxide which was very similar to the presently accepted one and which subsequent evidence cumulatively proved to be correct.
|
|
| H2O2 - gas phase (O-O 147.4 pm) |
|
| H2O2 - solid phase (O-O 145.8 pm) |
Although the O-O bond is a single bond, the molecule has a relatively high barrier to rotation of 29.45 kJmol-1 (2460 cm-1); for comparison, the rotational barrier for ethane is just 12.5 kJmol-1. The increased barrier is thought to be due to the repulsion between the lone pairs of the adjacent oxygen atoms.
The molecular structures of gaseous and crystalline H2O2 are significantly different. This difference is attributed to the effects of hydrogen bonding, which is absent in the gaseous state.
Preparation via the anthraquinone process
The anthraquinone process is a process for the production of hydrogen peroxide, which was developed by BASF. The industrial production of hydrogen peroxide is based on the reduction of oxygen with hydrogen, by the direct synthesis from the elements. Instead of hydrogen itself, however, a 2-alkyl-anthrahydroquinone (generated from the corresponding 2-alkyl-anthraquinone by catalytic hydrogenation with palladium) is used. Oxygen and the organic phase react to give hydrogen peroxide and the anthraquinone can be recycled.
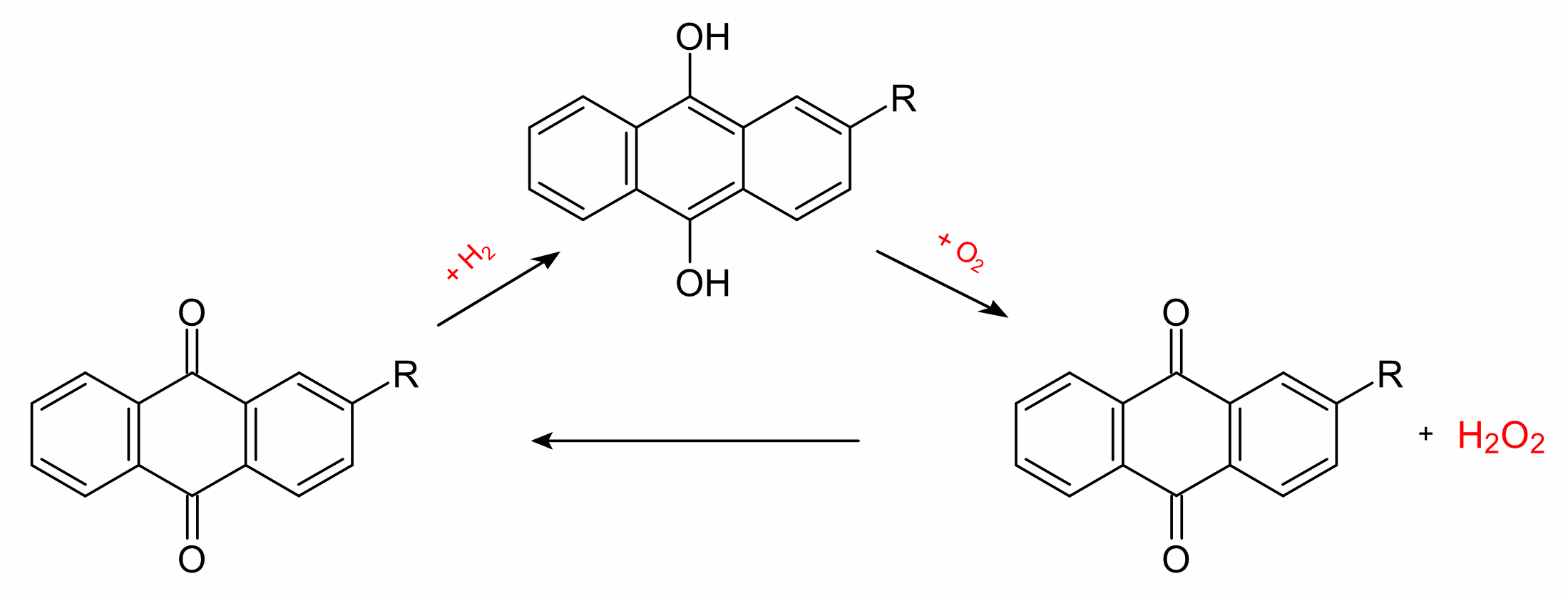 The hydrogen peroxide is then extracted with water in a second step and separated by fractional distillation. The anthraquinone thus acts as a catalyst with the overall reaction given by the equation:
The hydrogen peroxide is then extracted with water in a second step and separated by fractional distillation. The anthraquinone thus acts as a catalyst with the overall reaction given by the equation:
H2 + O2 → H2O2 If ozone is used instead of oxygen, dihydrogen trioxide, H2O3 can be produced by this method.
Decomposition
Hydrogen peroxide is thermodynamically unstable and decomposes to form water and oxygen with a ΔH⦵ of -98.2 kJmol-1 and a ΔS⦵ of 70.5 Jmol-1K-1.
2 H2O2 → 2 H2O + O2 The rate of decomposition increases with rising temperature, concentration and pH, with cool, dilute, acidic solutions showing the best stability. Decomposition is catalysed by various compounds, including most transition metals and their compounds (e.g. manganese dioxide, silver, and platinum). Certain metal ions, such as Fe2+ or Ti3+, can cause the decomposition to take a different path, with free radicals such as (HO.) and (HOO.) being formed.
The decomposition of hydrogen peroxide liberates oxygen and heat; this can be dangerous as spilling high concentrations of hydrogen peroxide on an inflammable substance can cause an immediate fire.
Metal oxides, peroxides and superoxides
When the group 1 metals are heated in an excess of air or in O2, the principal products obtained depend on the metal: lithium oxide, Li2O, sodium peroxide, Na2O2, and the superoxides KO2, RbO2 and CsO2.
4Li + O2 → 2Li2O oxide formation
2Na + O2 → Na2O2 peroxide formation
K + O2 → KO2 superoxide formation
The superoxides and peroxides contain the paramagnetic [O2]- (μ ~ 1.73 BM) and diamagnetic [O2]2- ions respectively. See the MO diagram for O2 above for comparison.
All of the monoxides are known, M2O, and the heavier peroxides and superoxides will decompose to form the oxides. They are all ionic and strong bases with the basicity increasing down the group.
O2- + H2O(aq) → 2 OH- (aq) Sodium peroxoborate is a solid peroxygen compound with exceptional storage stability and no shock sensitivity. It is cheap and readily available, being produced mainly as a solid ingredient of domestic washing formulations, in which it acts as sources of H2O2 in solution for stain bleaching. World annual production in 1995 was approximately 750,000 tonnes, and its use in washing powders dates back to 1907 with Henkel's original "Persil" product in Germany.
Sodium peroxoborate has the empirical formula "NaBO3.xH2O". Two forms that are commercially available correspond stoichiometrically to x = 1 or 4, and are known as the "monohydrate" and "tetrahydrate', respectively. Structurally, however, sodium peroxoborate was shown in 1961 to be the disodium salt of a 1,4.-diboratetroxane dianion. Hence, the "monohydrate" really corresponds to the anhydrous salt, and the "tetrahydrate" to a hexahydrated form of it. The monohydrate form dissolves better than the tetrahydrate and has higher heat stability; it is prepared by heating the tetrahydrate. The compound exists as a dimer as shown below.
The reagent offers low toxicity and a long shelf life. Sodium peroxoborate is a useful reagent in synthetic chemistry as a substitute for the unstable, highly concentrated hydrogen peroxide solutions that can pose a significant explosion hazard and are not commercially available.
Persil and the structure of Na2B2(O2)2(OH)4.6H2O
|
|
Return to the course outline or move on to Lecture 5: Structure of the elements (Groups 1 and 2 metals, Boron, Carbon and Phosphorus, Sulfur).
References
Much of the information in these course notes has been sourced from Wikipedia under the Creative Commons License.
'Inorganic Chemistry' - C. Housecroft and A.G. Sharpe, Prentice Hall, 4th Ed., 2012, ISBN13: 978-0273742753, pps 24-27, 43-50, 172-176, 552-558, 299-301, 207-212
'Basic Inorganic Chemistry' - F.A. Cotton, G. Wilkinson and P.L. Gaus, John Wiley and Sons, Inc. 3rd Ed., 1994.
'Introduction to Modern Inorganic Chemistry' - K.M. Mackay, R.A. Mackay and W. Henderson, International Textbook Company, 5th Ed., 1996.

