15.4B: Phosphorus
- Page ID
- 34218
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Phosphorus is found extensively non-crystalline phosphate rocks such as apatite, Ca3(PO4)2.CaX2 where X- = F- and/or Cl- and/or OH-.
Arsenic, antimony and bismuth come mainly from sulphide ores such as mispickel, FeAsS, and stibnite, Sb2S3.
- Apart from the hydrides such as phosphine, PH3, the elements are very different to nitrogen.
The differences arise from lower pp - pp bonding effectiveness replaced by use of the d-orbitals for dp - pp bonding, and gradual increasing stability of the lower oxidation states on descending the group:
- compare O=N-OR with P(-O-R)3.
- Compare the oxides of nitrogen with multiple bonding in all of them with P4O6 and P4O10 containing only single bonds.
- Compare nitric acid, HONO2, with pp - pp bonding with phosphoric acid, (HO)3PO, where phosphorus uses a d-orbital to form a p-bond to the oxygen (perhaps).
- Unlike nitrogen, the rest of the elements of group 15 can exceed 4-covalency, e.g. PF5, PF6- by using empty d-orbitals.
- As ligands with electron rich transition metals, all the group 15 elements, except nitrogen, can use their empty d-orbitals to act as p-acceptors (i.e. they are "soft").
The Elements
- Phosphorus, the most reactive can be obtained by carbon reduction of calcium phosphate:
Ca3(PO4)2 + 6SiO2 + 10C
 P4 + 6CaSiO3 + 10CO
P4 + 6CaSiO3 + 10COThe yellow phosphorus, P4 must be collected under water since it inflames in air.
- The other elements can be obtained by carbon reduction of their oxides.
- Reactivity:
- All the elements will react directly with oxygen especially if heated.
- The products of reaction with oxidizing acids, e.g. nitric acid illustrate the increasing metallic character down the group. The products are: H3PO4, H3AsO4, Sb4O6 and Bi(NO3)3. Note that the products are in the V oxidation state for phosphorus and arsenic, and the III-state for antimony and bismuth. Bismuth produces a nitrate salt.
- Compounds can be formed by direct reaction with other non-metals. One important compound is gallium arsenide, a semiconductor which is particularly heat resistant.
Hydrides
- The hydrides are all gases and increasingly unstable down the group. The classical (Sherlock Holmes style) test for arsenic is to generate AsH3 by reduction and observe the metallic arsenic mirror produced by decomposition of the gas on the glass of the test tube above the reaction mixture.
- The (Lewis) basic properties decrease down the group. There are some phosphonium, PH4+ compounds, but water tends to be a stronger base than PH3 so they tend to decompose in water.
Halides and Oxo Halides
- Refer to Figure 17-1 for some reactions of PCl3 which are typical of the group.
- Note that the solid state structures sometimes differ from the gas phase ones, for example, PCl5 is probably like PF5, a trigonal bipyramidal molecule in the gas phase, but in the solid state it is ionic: [PCl4]+[PCl6]-. PBr5, in the solid state, is [PBr4]+Br-.
- The Oxo trihalides, notably PCl3O, undergo reaction similar to the trihalides.
- SbOCl and BiOCl are obtained when hydrocloric acid solutions containing Sb3+ or Bi3+ are diluted.
- The pentaflourides are all good flouride ion acceptors to give non-coordinating anions such as AsF6- and the corresponding "super acids".
Oxides
- The oxides of the elements in the V state are most stable (relative to III) for phosphorus in addition to being the most acidic.
- "Phosphorus pentoxide" which is actually P4O10 is one of the most powerful dessicating agents known. It reacts with water to produce phosphoric acid, and can remove water from nitric acid, to give dinitrogen pentoxide and from sulphuric acid, itself a powerful dessicant to give sulphur trioxide.
Its structure is a tetrahedron of phosphorus atoms connected by oxygens on th esix edges of the tetrahedron and each carrying a terminal oxygen atom.
- The phosphorus III oxide, P4O6 is formed when phosphorus burns in a limited supply of oxygen. One of its forms is similar to P4O10 except that the terminal oxygen atoms are absent. It hydrolyses to phosphorous acid, H3PO3. Arsenic and Antimony produce similar compounds.
- The oxide and hydroxide of bismuth III, Bi2O3 and Bi(OH)3, obtained by adding base to solutions of Bi3+ salts, are not acidic at all.
Sulphides
- Some are somewhat related to the oxides but the numbers of terminal sulphurs varies more, others are chain or ribbon structures. Skip the details.
The Oxo Acids and their Esters
- The oxo acids have already been covered to some extent in chapter 5.
- Phosphorous acid is obtained by controlled hydrolysis of PCl3 or P4O6. Its formula is best written HP(O)(OH)2 to illustrate its structure with only two acidic hydrogens (on oxygen).
- Similarly, hypophosphorous acid is best written H2P(O)(OH). It is a monobasic acid.
- Phosphite esters, P(OR)3, are related to PX3 compounds. These compounds are easily oxidised to phosphate esters: OP(OR)3.
- The phosphite esters undergo the Michaelis-Arbusov reaction with alkyl halides:
P(OR)3 + R'X
 [(RO)3PR']+X-
[(RO)3PR']+X-  (RO)2(R')PO + RX
(RO)2(R')PO + RXThe dialkyl phosphonates produced are structurally similar to phosphorous acid where R replaces H.
- (Ortho)phosphoric acid, H3PO4, when pure is a solid melting at 42.5 oC. It i ssold as "syrupy phosphoric acid", an 85% solution in water. It is made by hydrolysing P4O10 or treating phosphate rocks with sulphuric acid. Its dehydration to pyrophosphoric acid (HO)2(O)POP(O)(OH)2 is slow.
- Phosphate esters are important in biochemical processes for energy storage and transfer. The mechanism of their hydrolysis has been extensively studied in this context.
Complexes of the Group 15 Elements
- Antimony forms some complexes, particularly with chelating oxy-ligands. One of the oldest know in the complex with tartrate, K2[Sb2(d-C4H2O6)2].3H2O known as "tartar emetic".
- Bismuth should behave much more like a true metal, but the simple aquo ion, [Bi(H2O)6]3+ does not seem to exist. There is a range of extensively hydrolysed clusters ions containing several bismuth III ions bridged by oxygen, and carrying OH groups. Skip the details!
Phosphorus-Nitrogen Compounds
- Six and eight membered ring systems and linear polymers containing ...-N=P-N=P-... chains can be generated by the reaction:
nPCl5 + nNH4Cl
 (NPCl2)n + 4nHCl (Solvents: C2H2Cl4 or C6H5Cl)
(NPCl2)n + 4nHCl (Solvents: C2H2Cl4 or C6H5Cl)Then the chlorine atoms can be replace by alkoxy, alkyl, or aryl groups by reaction with NaOR or LiR reagents. The resulting compounds can be made into useful fibres or elastomers.
Compounds with Element-Element Double Bonds
- Just as in group 14, therehave been attempts to stabilize compounds with multiple bonds betweemn the group 15 elements and just as in group 14, bulky groups get the job done. Molecules with double bonds between two phosphorus atoms, a phosphorus and an arsenic or two arsenic atoms have been sythesized using groups such as 2,4,6-(Me3C)3C6H2 and (Me3Si)3C have been used. The typical synthetic routes (where E = P or As) are:
2RPCl2 + 2Mg
 RP=PR + 2MgCl2
RP=PR + 2MgCl2or
RECl2 + H2E'R'
 RE=E'R' + 2HCl (In the presence of base)
RE=E'R' + 2HCl (In the presence of base)
Allotropes of phosphorus
Elemental phosphorus exists in a number of different allotropes.
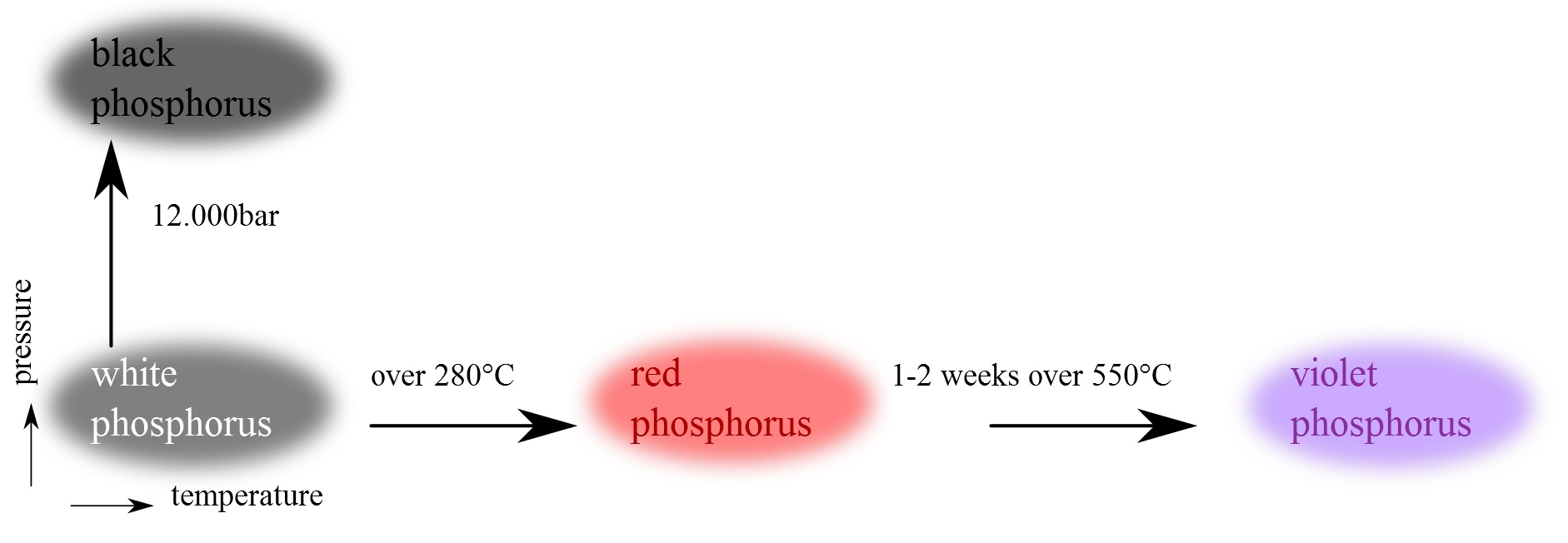 White phosphorus
White phosphorusThe most important form of elemental phosphorus from the perspective of applications and the chemical literature is white phosphorus. It consists of tetrahedral P4 molecules, in which each atom is bound to the other three atoms by a single bond. This P4 tetrahedron is also present in liquid and gaseous phosphorus up to the temperature of 800 °C when it starts decomposing to P2 molecules. Solid white phosphorus exists in two forms. At low-temperatures, the β form is stable. At high-temperatures the α form is predominant. These forms differ in terms of the relative orientations of the constituent P4 tetrahedra.
The history of the match is linked to the discovery of the allotropes of phosphorus.
|
|
white |
|
| red |
|
| violet - Hittorf |
|
| black |
|
|
| Phosphorene is an allotrope of phosphorus normally used to designate a single layer of black phosphorus that may be somewhat flattened. Conceptually the structure is similar to the carbon-based graphene, hence the name phosphorene. However phosphorene is a semiconductor, unlike graphene which is a semimetal. Recently a sample that was about 20 layers thick was shown to demonstrate high-speed data communication on nanoscale optical circuits. |
Red phosphorus
In 1847 Anton von Schrotter found that sunlight changed white/yellow into red phosphorus, even when moisture and atmospheric oxygen were rigorously excluded. The red product was separated from the residual yellow phosphorus by treatment with carbon disulfide. Red phosphorus was also prepared from the yellow variety by heating it to about 250 °C. in an inert gas. Heating to higher temperatures reconverted the red modification to the yellow one.
Red phosphorus exists as an amorphous network and does not ignite in air at temperatures below 240 °C.
Violet phosphorus
In 1865, Johann Hittorf heated red phosphorus in a sealed tube at 530 °C. The upper part of the tube was kept at 444 °C. Brilliant opaque monoclinic, or rhombohedral, crystals sublimed.
This form is sometimes known as "Hittorf's phosphorus" (or violet or α-metallic phosphorus).
Black phosphorus
Black phosphorus is the thermodynamically stable form of phosphorus at room temperature and pressure. It is obtained by heating white phosphorus under high pressures (12,000 atmospheres). In appearance, properties and structure it is similar to graphite, being black and flaky, a conductor of electricity, and having puckered sheets of linked atoms.
Black phosphorus has an orthorhombic structure and is the least reactive allotrope: a result of its lattice of interlinked six-membered rings. Each atom is bonded to three other atoms.

