6.11A: Structure - Rock Salt (NaCl)
- Page ID
- 2589
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Rock salt also known as NaCl is an ionic compound. It occurs naturally as white cubic crystals. The structure of NaCl is formed by repeating the unit cell. It has an organized structure and has a 1:1 ratio of Na:Cl.
Introduction
Rock salt (\(\ce{NaCl}\)) is an ionic compound that occurs naturally as white crystals. It is extracted from the mineral form halite or evaporation of seawater. The structure of NaCl is formed by repeating the face centered cubic unit cell. It has 1:1 stoichiometry ratio of Na:Cl with a molar mass of 58.4 g/mol. Compounds with the sodium chloride structure include alikali halides and metal oxides and transition-metal compounds. An important role to many important applications is structure and dynamics of water. Some applications include crystallization of proteins and conformational behavior of peptides and nucleic acids.
Structure
Figure \(\PageIndex{1}\) shows how the Na+ and Cl- ions occupy the space. The smaller ions are the Na+ with has an atomic radius of 102 pm, and the larger ions are the Cl- with an atomic radium of 181 pm. Since NaCl are one to one ratio as a compound, the coordination numbers of Na and Cl are equal. The larger green ions represent Cl- and the smaller purple ions represent Na+. However, the structure of this molecule allows their positions to be switched since the coordination numbers are equivalent.
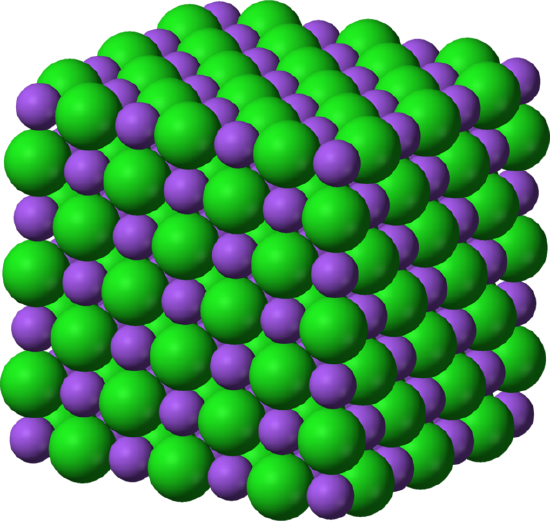
A Unit Cell
The unit cell of \(\ce{NaCl}\) consists of \(\ce{Na^{+}}\) ions and \(\ce{Cl^{-}}\) ions. There are four types of site: unique central position, face site, edge sites and corner site, which are used to determine the number of Na+ ions and Cl- ions in the unit cell of NaCl. When counting the number of ions, a corner site would be shared by 7 other unit cells. Therefore, 1 corner would be 1/8 of an ion. A similar occurrence happens with the face site and the edge sites. For a face site, it is shared by 1 other unit cell and for an edge site, the ion is shared by 3 other unit cells. \(\ce{NaCl}\) is a face centered cubic unit cell which has four cations and four anions. This can be shown by counting the number of ions and multiplying them in relation to their position.
- \(\ce{Na^{+}}\): \[1_{center} + 12_{edge} \times \dfrac{1}{4} = 4\, \text{sodium ions total per cell} \nonumber\]
- \(\ce{Cl^{-}}\): \[4_{face} \times \dfrac{1}{2} + 8_{corner} \times \dfrac{1}{8} = 4\, \text{chloride ions total per cell} \nonumber\]
Each ion in this lattice has six of the other kind of ion as its nearest neighbors, and twelve of the same kind of ions as its second nearest neighbors. There are many ionic compounds that assume this structure including all other halides of Na, Li, K and Rb. CsF, AgF, AgCl, BaO, CoO, and SrS are also among many that will form similar structures to NaCl.
Outside Links
- Video about structure of NaCl: http://www.youtube.com/watch?v=csfOBynrF8E
- Unit Cell. http://www.case.edu/artsci/chem/chim...ids/xtal1.html
References
- Gao, H.X., L.-M. Peng, and J.M Zuo. "Lattice dynamics and Debye-Waller factors of some compounds with the sodium chloride structure." Acta Crystallographica: Section A (Wiley-Blackwell) 55.6 (1999): 1014. Academic Search Complete. EBSCO. Web.
- Housecroft, Catherine E., and Alan G. Sharpe. Inorganic Chemistry. 3rd ed. Harlow: Pearson Education, 2008. Print.
- Jun Soo, Kim, and Yethiraj Arun. "A Diffusive Anomaly of Water in Aqueous Sodium Chloride Solutions at Low Temperatures." Journal of Physical Chemistry B 112.6 (2008): 1729-1735. Academic Search Complete. EBSCO. Web.
Contributors and Attributions
- Michael Ford

