6.5: Quantum Numbers
- Page ID
- 408814
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Quantum numbers
Every electron localized in an atom can be described by four quantum numbers. The Pauli Exclusion Principle tells us that no two electrons can share the exact same set of quantum numbers
Principal quantum number
The principle quantum number, \(n\), represents the energy level of the electron, much like the \(n\) used in the Bohr model. Energy is related to the principle quantum number by
\(E=-\dfrac{13.6 \mathrm{eV}}{n^2}, \quad n>0\)
Orbital angular momentum quantum number
The orbital angular momentum quantum number, \(l\), provides information about the shape of an orbital. Unlike the description of early models of the atom, electrons in atoms don't orbit around the nucleus like a planet around the sun. However, they do have angular momentum, and the shape of the probability cloud around the nucleus depends on the value of the angular momentum. The magnitude of the angular momentum is related to the orbital angular momentum quantum number by
\(L=\hbar \sqrt{l(l+1)}, \quad 0 \leq l \leq n-1\)
The orbital angular momentum quantum numbers correspond directly to letters commonly used by chemists to describe the orbital subshells: for example, \(l=1\) corresponds to the \(\mathrm{s}\) orbitals, which are spherical:

\(l=2\) corresponds to the \(\mathrm{p}\) orbitals, which are lobed: Visualizations of the higher orbitals, like the \(\mathrm{d}(l-2)\)
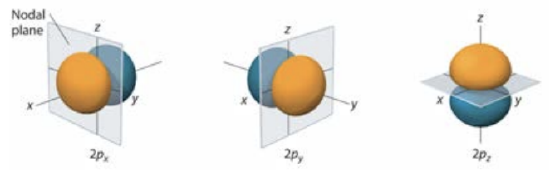
and \(f (l = 3)\) can be found in Chapter 6 of the Averill textbook.
Magnetic quantum number
The magnetic quantum number, \(m\), distinguishes the orbitals available within a subshell. The projection of the angular momentum onto the \(\mathrm{z}\)-axis is related to the magnetic quantum number by
\(L_z=m \hbar, \quad-l \leq m \leq l\)
Spin quantum number
The spin quantum number, \(m_s\), gives the spin angular momentum of each electron. Each electron can either be spin up or spin down. The magnitude of the spin is either \(+1 / 2\) (spin up) or \(-1 / 2\) (spin down). The magnitude of the angular momentum associated with spin is
\(|S|=\hbar \sqrt{s(s+1)}\)
and the spin projection onto the \(\mathrm{z}\)-axis is given by
\(S_z=s \hbar\)
The restriction on the allowed values of spin is
\(s=\pm \dfrac{1}{2}\)
What is the electronic configuration of carbon? Write out the quantum numbers for each electron.
Solution
Carbon has six valence electrons. We'll start filling up the quantum numbers by following the rules above. Starting with \(n=1\), the only possible value of \(l\) is \(l=0\), since \(0 \leq l \leq n-1\). When \(l=0, m\) must also be 0 . There are two possible spins that could go with these quantum numbers. There aren't any more available quantum numbers with \(n=1\), so we must go up to a higher shell. For \(n=2\), the lowest energy state corresponds to the lowest magnitude of angular momentum, \(l=0\), which again requires \(m=0\).
For the last two electrons, we have to remember Hund's rule. Every orbital in a subshell must be singly occupied with electrons of one spin before adding electrons of the other. So the \(l=1\) electrons must have different values of \(m\), but the same value of \(m_s\). The quantum numbers are summarized in the table below.
\[\begin{array}{c|c|c|c}
n & l & m & m_s \\
\hline \hline 1 & 0 & 0 & 1 / 2 \\
1 & 0 & 0 & -1 / 2 \\
2 & 0 & 0 & 1 / 2 \\
2 & 0 & 0 & -1 / 2 \\
2 & 1 & 0 & 1 / 2 \\
2 & 1 & -1 & 1 / 2
\end{array} \nonumber\]


