2.8: Self-Assessment- Reactions and Kinetics + Answer
- Page ID
- 408601
1. Urbium (Ur) is an upscale element found in big cities. Its oxide \(\left(\mathrm{UrO}_2\right)\) is not very stable and decomposes readily at temperatures exceeding \(666^{\circ} \mathrm{C}\). The figure below shows how the rate of reaction varies with the concentration of \(\mathrm{UrO}_2\) at \(777^{\circ} \mathrm{C}\). The rate, \(r\), is in units of \(\mathrm{M} / \mathrm{s}\) and the concentration of \(\mathrm{UrO}_2, c\), is in units of \(\mathrm{M}(\mathrm{mole} / \mathrm{L})\). The slope has a value of \(1.77\) and the intercept has a value of \(1.46\).
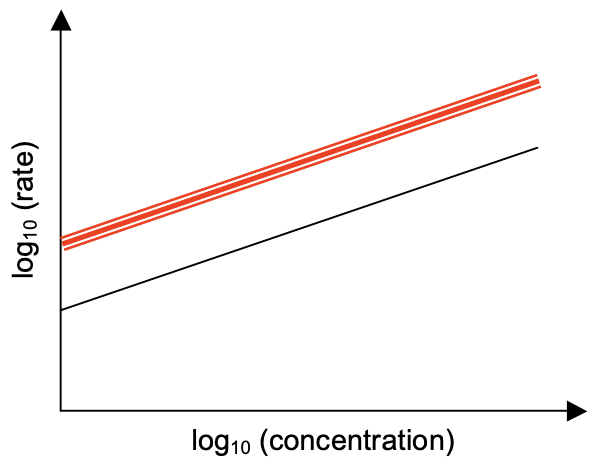
(a) What is the order of reaction?
- Answer
-
The order is the slope: 1.77
(b) Calculate the value of the rate constant. Pay strict attention to the units.
- Answer
-
\(r=k c^n \rightarrow \log r=\log k+n \log c ; \text{when } c=1, r=k=10^{1.46}=28.8\)
\(\text{Units of } k=r / c^{\mathrm{n}}=(\mathrm{M} / \mathrm{s}) /\left(\mathrm{M}^{1.77}\right)=\mathrm{M}^{-0.77} / \mathrm{s} \rightarrow k=28.8 \mathrm{M}^{-0.77} / \mathrm{s}\)
(c) On the graph above, draw the line showing how the rate of reaction varies with the concentration of \(\mathrm{UrO}_2\) at \(888^{\circ} \mathrm{C}\). No calculation necessary. Pay attention to relative values and slopes.
- Answer
-
The upper line on the graph represents the isotherm at \(888^{\circ} \mathrm{C}\). Note same slope as \(777^{\circ} \mathrm{C}\) but greater value of \(r\)-intercept.
2. Show by a calculation that the diffusion length of boron (B) in germanium (Ge) is less than \(1.0 \mu \mathrm{m}\) at a temperature of \(1200 \mathrm{~K}\) for a diffusion time of 30 minutes. The diffusion coefficient of B in Ge at \(1200 \mathrm{~K}, D_{\mathrm{B}}\), has the value of \(2.0 \times 10^{-17} \mathrm{~m}^2 / \mathrm{s}\).
- Answer
-
The diffusion length is approximated by the relationship \(x=\sqrt{D t}\) or \(x=2 \sqrt{D t}\)
\(\therefore \sqrt{D t}=\sqrt{2.0 \times 10^{-17} \frac{\mathrm{m}^2}{\mathrm{~s}} \cdot 30 \mathrm{~min} \cdot 60 \frac{\mathrm{s}}{\mathrm{min}}}=1.90 \times 10^{-7} \mathrm{~m}<1.0 \mu \mathrm{m}\)


