5.2: Nomenclature
- Page ID
- 275344
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Classification of Ligands
Let us look a little closer at ligands and see how we can classify them. One way is to categorize them into monodentate and multi-dentate ligands.
Monodentate ligands have only one point of attachment to the metal ion.
Definition: Monodentate ligand
Monodentate ligands have only one point of attachment to the metal ion
Examples for such ligands are halogenide ligands, ammonia as ligand, and water as ligand. The molecules often have different names when acting as ligands, and you must know these names. For example, water as a ligand is called an aqua ligand, ammonia as a ligand is called an ammine ligand, chloride as a ligand is called a chloro ligand.
Ligands with more than one point of attachment are called multidentate ligands, or chelate ligands.
Definition: Multidentate ligands
Multi-dentate ligands have two or more points of attachment to the metal ion
Complexes with chelate ligands are called chelate complexes. Greek prefixes indicate how many points of attachment the ligand has (Fig. 5.2.1).

If there are two, then we have a bidentate ligand, when there are three, we have a tridentate ligand. We use the prefixes tetra, penta, and hexa to indicate four, five, and six points of attachment, respectively. Multi-dentate ligands with more than six points of attachment are rare.
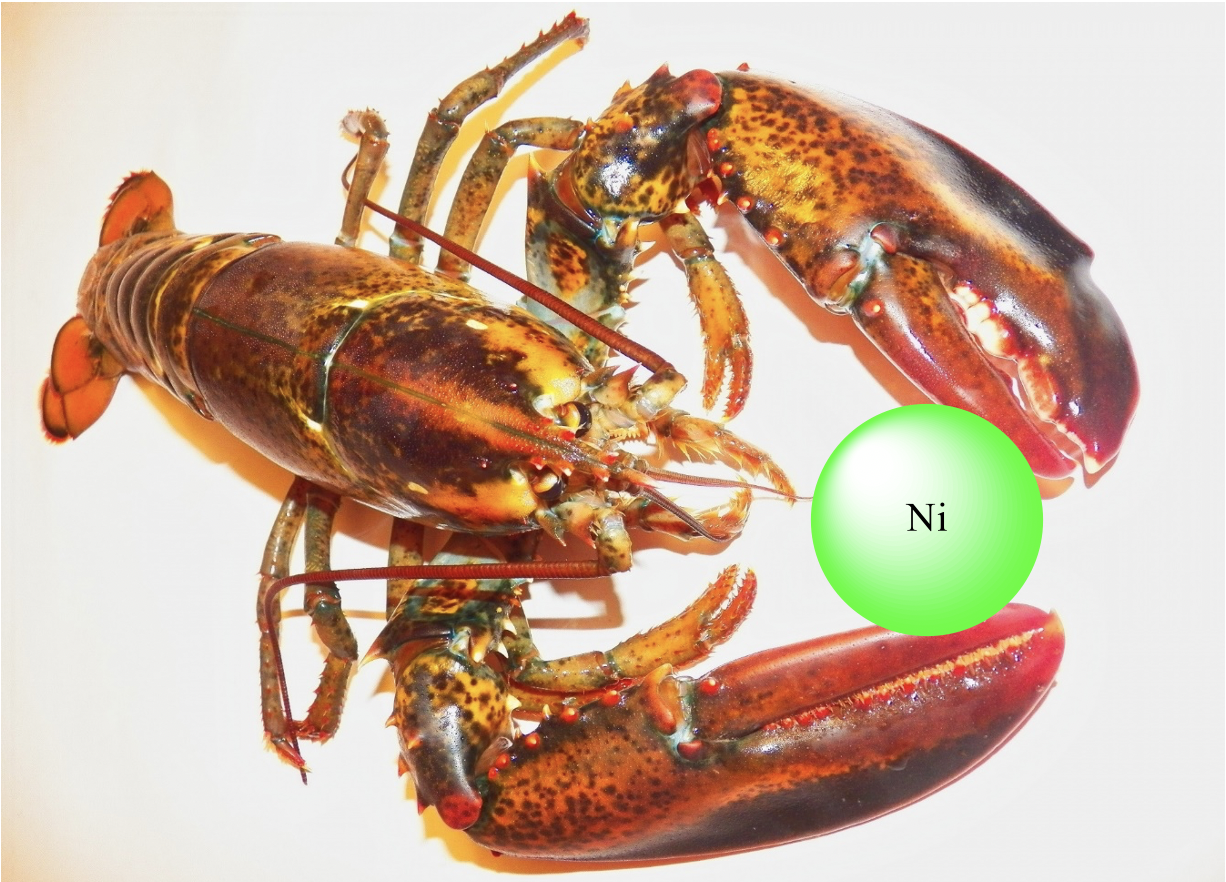
The name chelate ligand comes from the Greek word chela, meaning great claw of the lobster. We can see that our lobster in Figure 5.2.2 happily chelates a Ni2+ ion with its two great claws!
Common Bidentate Ligands
A few common bidentate ligands are shown below.
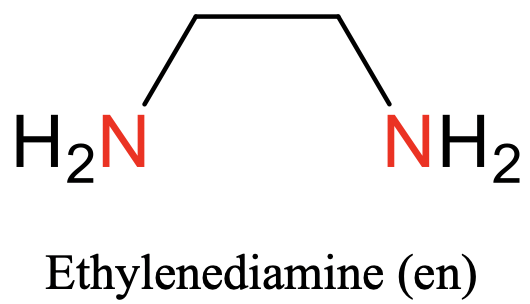
The first one is ethylene diamine. It has two nitrogen donor atoms that can bind to the metal, and they are separated by an ethylene group (Fig. 5.2.3).
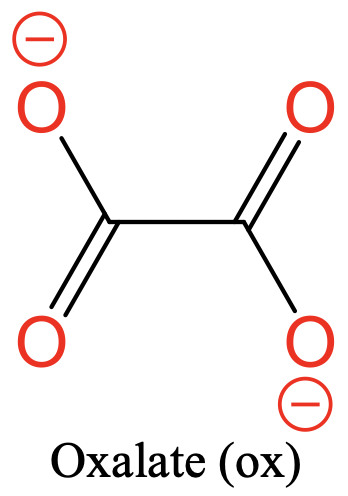
Another common ligand is the oxalate ligand. It has two O donor atoms separated by two carbon atoms. There are two negative charges that are delocalized over the four O atoms (Fig. 5.2.4).
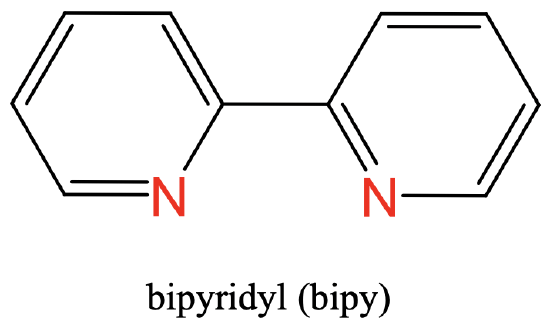
As a third example you can see the bipyridyl ligand having two N donor atoms as part of two aromatic rings. The two N atoms are separated by two carbon atoms (Fig. 5.2.5).
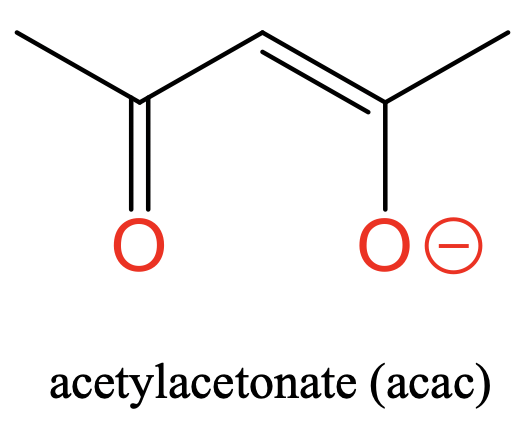
Lastly, there is the acetyl acetonate ligand with two O donor atoms separated by three C atoms (Fig. 5.2.6). The acetyl acetonate carries a negative charge that is delocalized between the two O atoms. In this case the donor atoms are separated by three carbons.
Common ligands often have specific abbreviations. They are often used in the formulas of coordination compounds with these ligands. For example, the ethylene diamine ligand is abbreviated en, the oxalate ligand is abbreviated ox, the bipyridyl ligand is abbreviated bipy, and the acetyl acetonate ligand is abbreviated acac. There are not only bidentate ligands with O and N donor atoms, but also with others such as P and S.
Rings in Complexes with Chelate Ligands
In most chelating ligands the donor atoms are separated by two or three other atoms, mostly carbon atoms. This is because in this case the chelate ligands can form five- and six-membered rings with the metal ion (Fig. 5.2.7).
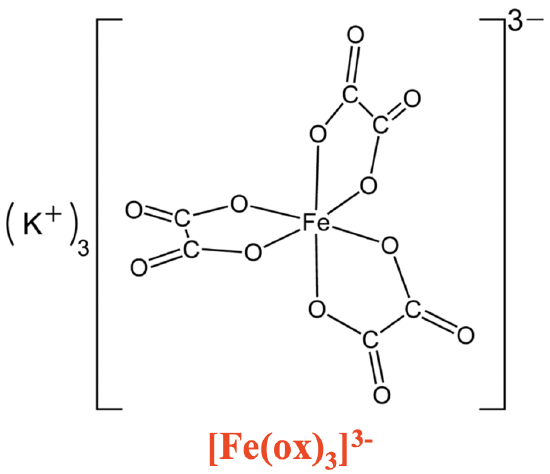
You can see that the trioxolato iron complex above has three five-membered rings containing one Fe, two O, and two C atoms. These ring sizes are particularly stable because they have the least ring strain. As a consequence, the respective chelate complexes are particularly stable.
Tridentate Ligands
Here are a couple of examples for tridentate ligands.
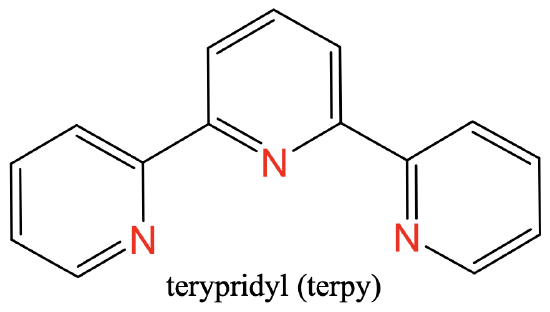
The first one is terpyridyl, abbreviated “Terpy”(Fig. 5.2.8), the second one is bisethylenetriamine, abbreviated “tris” (Fig. 5.2.9).
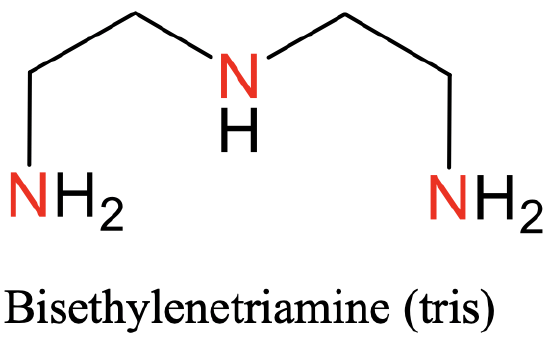
Both of them have three N-donor atoms separated by two carbon atoms. In the “tris”-ligand there are two ethylene groups between the N atoms, in the case of the “terpy”-ligand the N-atoms are part of three aromatic rings (Fig. 5.2.8. and Fig. 5.2.9)
Tetradentate Ligands
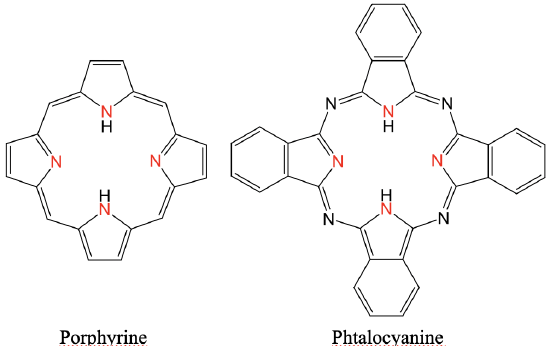
Two common tetradentate ligands are porphyrine and phtalocyanine (Fig. 5.2.9 and 5.2.10). Both are co-called macrocyclic ligands because they are large cycles. They both have four N-donor atoms pointing inside of the cycle. The phtalocyanine ligand has four additional N atoms connecting the five-membered rings via imine-linkages. Further the phtalocyanine has four benzene rings fused to the four five-membered rings. The porphyrine ligand is very important in biological systems. For example, it is part of the protein hemoglobin. In this case a an Fe2+ ion sits in the center of the porphyrin ring. It is also a component of chlorophyll in which case an Mg2+ ion sits in the center of the ring. Phtalocyanine ligands are important as components of dyes.
Hexadentate Ligand, EDTA
A very common hexadentate ligand is the ethylenediamine tetraacetic acid (EDTA) ligand. You can see its structure below (Fig. 5.2.11).
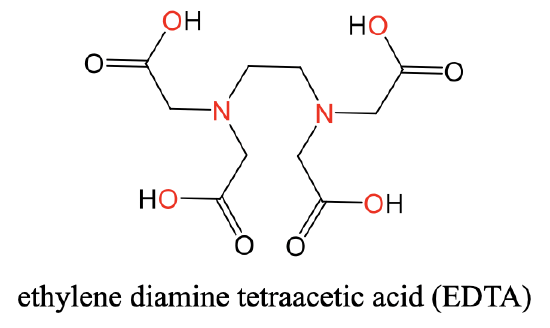
It has two N-donor atoms separated by an ethylene group. Each N-atom is further connected to two acetyl groups. The overall four acetyl groups carry four O-donor atoms. Overall, there are six donor atoms. The six donor atoms can coordinate octahedrally to a metal ion such as a Ca2+ ion (Fig. 5.2.12).
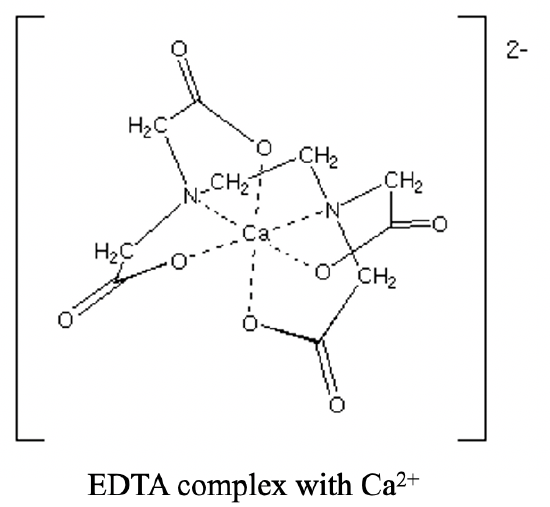
In coordinated form the EDTA ligand is deprotonated, and the O donor atoms carry a negative charge. Therefore an EDTA complex with a divalent cation such as Ca2+ has a 2- charge.
Nomenclature of Complexes
Now let us develop a nomenclature for coordination chemistry so that we can communicate them in an educated manner. One important aspect is that we name the number of ligands. To indicate the number of a particular ligand we use Greek prefixes (Fig. 5.2.13).
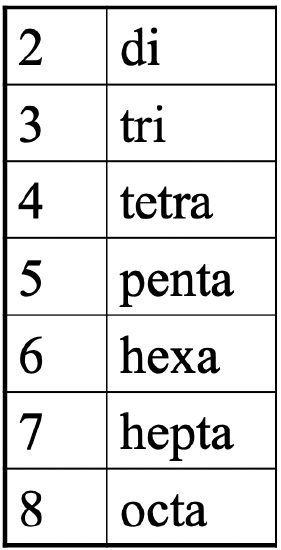
These are the same prefixes we got to know when we discussed multidentate ligands. If there are two ligands we use the prefix di, if there are three we use the prefix tri- and so fourth.
Nomenclature of Complexes with Anionic Coordination Spheres
We can now develop the full name of a coordination compound. Let us consider compounds with complex anions first. We can name them according to three steps.
In the first step we name the counter cation. We do not account for the number of counter cations in the name.
Next, we determine the name and the number of ligands. If the ligand is anionic it gets the suffix “o”. Note that sometimes for ease of pronunciation abbreviations are used. For example a Cl- is a chloro-ligand, and not a chlorido ligand. You must memorize these shorter forms. Generally, when an anion ends with “ide”, the “ide” is omitted, and replaced by “o”.
The third step is to name the metal ion and add the suffix “ate” to the name. You can either add the oxidation number of the metal in roman numerals or the charge of the complex anion in parentheses after the name of the metal. The first nomenclature is called the “Stock” system, the latter the “Ewing-Bassett” system. Note that if the element symbol of the metal is derived from a latin name then the latin name is used. For example if silver is the metal then the complex anion is an argentate, if lead is the metal the complex anion is a plumbate. Also here abbreviations are often used to make pronunciation easier. If the name ends with “um” that ending is replaced by “ate”.
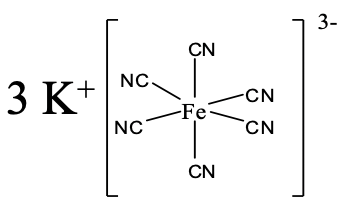
An example for coordination compounds with a complex anion is shown above (Fig. 5.2.14). What is its name? There are three K+ cations, so the name starts with potassium. We realize next, that there are six cyanide anions as ligands, so the name continues “hexacyano”. The name of the metal is iron, but we use the latin name ferrum, and replace the ending “um” with the ending “ate”. The oxidation number of the iron is +3. We can see that from the fact that the complex ion has a 3- charge, and the six cyano ligands have a 1- charge each. If we used the Stock system we would therefore place the roman numerals for +3 in parentheses behind the name. If we used the Ewing-Bassett system, we would place the (3-) for the negative charge of the complex behind the name. So overall it would be either a potassium hexacyanoferrate (III) or a potassium hexacyanoferrate (3-).
Exercises
How would you name the following two compounds?
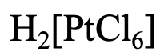
Let us look at the first example (Fig. 5.2.17). First, we need to name the cation. What is it? It is just “hydrogen”. Next we need to determine the name and number of ligands. We have six chloro ligands, so the name continues with “hexachloro”. The name of metal is platinum. We replace the ending “um” by “ate”. In the Stock system the roman numeral would be (IV) because the oxidation state of the Pt is +4. We can see this from the fact that the complex anion has a 2- charge, and the six chloro ligands have a 1- charge each. We must add +4 to -6 to get to -2. Therefore, in the Stock system, the name would be hydrogen hexachloroplatinate (IV), and in the Ewing-Bassett system it would be hydrogen hexachloroplatinate (2-).
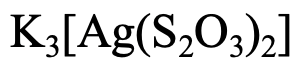
The second example (Fig. 5.2.18) has three K+ cations, so the name starts with potassium. What is the name of the ligand? The name of the anion is thiosulfate. In this case we replace the ending “e” by the ending “o”. We have two ligands, so it is a “dithiosulfato”. The metal is silver, but we use the latin name “argentum”, and replace the ending “um” by the ending “ate”. So it is an “argentate” . Fusing the parts together gives “potassium dithiosulfato argentate”. The oxidation number of Ag is +1, the charge at the anion is -3. So put either (I) in roman numerals or 3- in parentheses behind the name.
Nomenclature of Complexes with Cationic Coordination Spheres
Now let us name complexes with complex cations. We name the complex cation first, and then the anion. Then, we determine the name and the number of the ligands and prefixes accordingly. If there is an anionic ligand we give it the suffix “o” again. Then, we name the metal. In this case, we always use English names. We place the oxidation number in roman numerals or the charge of the complex cation behind the name, depending on whether we want to use the Stock or the Ewing-Bassett system.
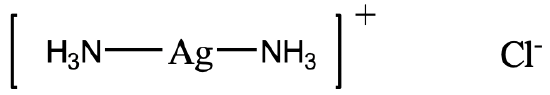
For example, how would you name the complex depicted above (Fig. 5.2.19)? There are two NH3 ligands which are neutral. We have to consider that NH3 as a ligand is called an “ammine” ligand. Note that it is spelled with two “m” in the middle. So the name starts “diammine”. The name of the metal is silver, and the oxidation state of silver is +1. This is because the complex has a 1+ charge and the ammine ligands are neutral. The anion is a chloride anion.
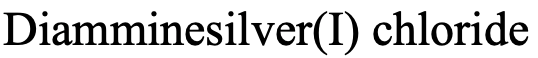
Therefore, the name in the Stock system would be “diamminesilver(I) chloride (Fig. 5.2.20).
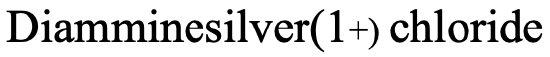
In the Ewing-Bassett system it would be “Diamminesilver (1+) chloride” (Fig. 5.2.21)
Exercises
What would be the names of the two compounds listed below.
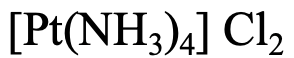
The first example, Fig. 5.2.22, has four NH3 ligands, so the name starts with tetraammine. Platinum is the metal, so the name continues “platinum”. The oxidation state of Pt is +2 because the complex cation has a 2+ charge, and the ligands are charge-neutral. The name of the anion is “choride”. So overall it is a “tetraammineplatinum(II) chloride” according to the Stock system or a “tetrammineplatinum(2+) chloride according to the Ewing-Bassett system.
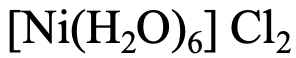
The second compound (Fig. 5.2.23) has six water ligands, therefore the name starts with “hexaaqua” followed by the name of the metal with is “nickel”. The oxidation state of Ni is 2+ because the charge at the complex cation is +2, and the ligands are charge-neutral. The name of the anion is “chloride”. Therefore, the name is either hexaaaqua nickel (II) chloride or “hexaaquanickel (2+) chloride.
Nomenclature of Complexes with Cationic and Anionic Coordination Spheres
There is also the possibility that a coordination compound is made of a complex cation and a complex anion. In this case, the rules discussed previously hold, the only new thing to learn is that we name the complex cation first and the complex anion second.
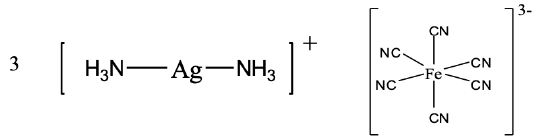
In the compound depicted (Fig. 5.2.24) we have a diammine silver (I) cation and a hexacyanoferrate (III) anion.
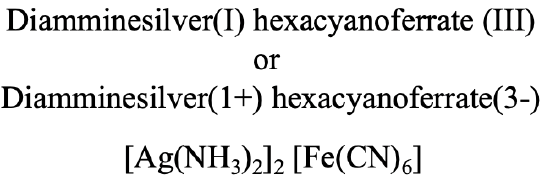
Hence the name is diammine silver (I) hexacyanoferrate (III) in the Stock system or diammine silver(1+) hexacyanoferrate (3-), Fig. 5.2.24.
Nomenclature of Complexes with More Than One Ligand of the Same Kind
What if there are different ligands in a coordination compound? In this case, we name the ligands in alphabetical order, and give each ligand a prefix according to its number.
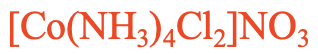
For example: What is the name of [Co(NH3)4Cl2]NO3 , Fig. 5.2.26? This is a compound with a complex cation containing ammine and chloro ligands. Because “a” comes before “c” in the alphabet we have to name the ammine ligand first. There are four ammine ligands and two chloro ligands. Therefore, we use the prefixes “tetra” in front of “ammine” and “di” in front of “chloro”. So the name starts “tetraamminedichloro”. Then, we name the metal which is cobalt. The oxidation number of cobalt is +3 because there are four charge-neutral ammine ligands, two anionic chloro ligands, and the charge at the complex cation is +1. +3 -2 = +1. So in the Stock system the compound is called tetramminedichlorocobalt(III) nitrate, in the Ewing-Basset system it is called tetramminedichlorocobalt(1+) nitrate.

Let us do one more example, Fig. 5.2.27. In the compound [Pt(NH3)BrCl(H2O)]SO4 there is a complex cation with four different ligands: an “ammine” ligand, a “bromo”-ligand, a "chloro"-ligand, and an "aqua" ligand. What is the order of them? According to the alphabet, “ammine” comes first, “aqua” is second, “bromo” is third, and “chloro” is fourth. They do not get a prefix because there is just one of them for each. The metal is platinum, and its oxidation state is +4 because the complex cation has a 2+ charge, and there are two neutral ligands, namely the aqua and the ammine ligands, and two anionic ones, namely the bromo and the chloro ligands: +4 - 2 = +2.

Therefore, the name is ammineaquabromochloroplatinum(IV)sulfate in the Stock system, and ammineaquabromochloroplatinum(2+)sulfate in the Ewing-Bassett system, 5.2.28.
Nomenclature of Complexes with Complicated Ligands in a Coordination Sphere
So far we only considered relatively simple ligands that were either monoatomic, or contained a few atoms only. However, many ligands, in particular chelating ligands, contain more atoms, and have more complex names. These names may already contain prefixes that we use to number ligands. For example the ethylenediamine ligand is a chelating ligand with a longer name that already contains the prefix “di”. In such cases, to avoid ambiguity, we put the ligand name in parentheses, and place a somewhat different prefix in front of it to account for the number of the ligands.

Instead of “di” we use “bis”, instead of “tri” we use “tris”, instead of “tetra” we use “tetrakis”, instead of “penta” we use “pentakis”, and so on, Fig. 5.2.29.
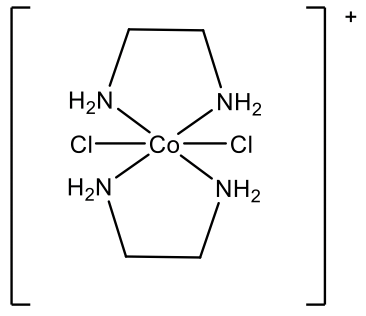
For example, what is the name of the complex cation depicted above (Fig. 5.2.30)? We first need to realize that there are two different ligands: chloro ligands and ethylenediamine ligands. We need to name the chloro ligands first, because they come first in the alphabet. Because there are two chloro ligands we use the prefix “di”. The ethylenediamine ligand is placed in parentheses, and the prefix “bis” is used instead of “di”. The metal is cobalt in the oxidation state +3 because the complex cation has a 1+ charge, and there are two chloro ligands with a 1- charge each, and two charge-neutral ethylenediamine ligands: +3 -2 = +1.

Therefore, the name is dichlorobis(ethylenediamine)cobalt(III) in the Stock system and dichlorobis(ethylenediamine)cobalt(1+) in the Ewing-Bassett system (Fig. 5.2.31).
Nomenclature of Complexes with Bridging Ligands
Ligands cannot only be terminal, they can also bridge two metal centers. To indicate that a ligand is a bridging ligand we give it a prefix μ. Bridging ligands are named before terminal ligands, and the name of the molecular fragments with the terminal ligands and metal ions is placed in parentheses after the name of the bridging ligand.
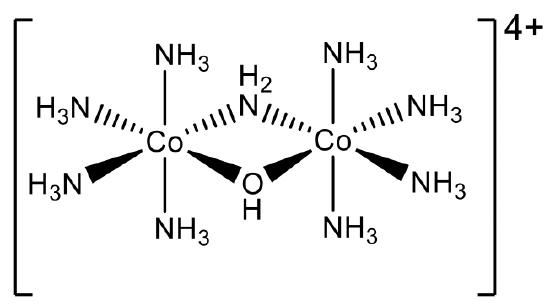
So what is the name of the complex cation depicted above (Fig. 5.2.32)? We can see that there are two different bridging ligands, one is a hydroxo-ligand OH-, and the other is called an amido ligand NH2-. These bridging ligands bridge two identical molecular fragments containing a Co atom and four ammine ligands each. The two bridging ligands need to be named first, and we have to name them according to alphabetic order. Hence, the name starts “ μ-amido- μ-hydroxo”. Then, we need to name the two fragments which are bridged. Because there is one Co and four NH3 ligands, the name of the fragment is “tetraamminecobalt”. Because this is a more complex name we need to put it into parentheses. There are two fragments, therefore we need to use the prefix “bis”. The oxidation state of Co is +3. This is because there are two anionic ligands, the hydroxo, and the amido ligand which have both a 1- charge. The other ligands are neutral. The complex cation carries a 4+ charge: +6-2=+4. Because there are two Co atoms, they have an oxidation number of +6/2=+3.

Hence, the overall name according to the Stock system is “ μ-amido-μ-hydroxobis(tetraamminecobalt)(III)”, Fig. 5.2.33.

In the Ewing-Bassett system it is μ-amido- μ-hydroxobis(tetraamminecobalt)(4+), Fig. 5.2.34. Now we have completed the chapter on nomenclature.
Dr. Kai Landskron (Lehigh University). If you like this textbook, please consider to make a donation to support the author's research at Lehigh University: Click Here to Donate.


