4.6: Halogens and Halides
- Page ID
- 125396
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The origin of halogen is the Greek word meaning the production of salt by direct reaction with a metal. Since their reactivity is very high, halogens are found in nature only as compounds. The basic properties of halogens are shown in Table \(\PageIndex{6}\) and Table \(\PageIndex{7}\). The electron configuration of each halogen atom is ns2np5, and they lack one electron from the closed-shell structure of a rare gas. Thus a halogen atom emits energy when it gains an electron. Namely, the enthalpy change of the reaction $$X\; (g) + e^{-} \rightarrow X^{-}\; (g)$$is negative. Although electron affinity is defined as the energy change of gaining an electron, a positive sign is customarily used. In order to be consistent with the enthalpy change, a negative sign would be appropriate.
| Ionization energy (kJ mol-1) |
Electronegativity \(\chi_{P}\) |
Ionic radius r(X-) (pm) |
|
|---|---|---|---|
| F | 1680.6 | 3.98 | 133 |
| Cl | 1255.7 | 3.16 | 181 |
| Br | 1142.7 | 2.996 | 196 |
| I | 1008.7 | 2.66 | 220 |
| Interatomic distance r(X-X) (pm) |
mp °C |
bp °C |
Color | |
|---|---|---|---|---|
| F2 | 143 | -218.6 | -188.1 | Colorless gas |
| Cl2 | 199 | -101.0 | -34.0 | Yellow green gas |
| Br2 | 228 | -7.75 | 59.5 | Dark red liquid |
| I2 | 266 | 113.6 | 185.2 | Dark violet solid |
The electron affinity of chlorine (348.5 kJ mol-1) is the largest and fluorine (332.6 kJ mol-1) comes between chlorine and bromine (324.7 kJ mol-1). The electronegativity of fluorine is the highest of all the halogens.
Since halogens are produced as metal salts, simple substances are manufactured by electrolysis. Fluorine only takes the oxidation number -1 in its compounds, although the oxidation number of other halogens can range from -1 to +7. Astatine, At, has no stable nuclide and little is known about its chemical properties.
(a) Manufacture of halogen
Fluorine has the highest reduction potential (E = +2.87 V) and the strongest oxidizing power among the halogen molecules. It is also the most reactive nonmetallic element. Since water is oxidized by F2 at much lower electrode potential (+1.23 V), fluorine gas cannot be manufactured by the electrolysis of aqueous solutions of fluorine compounds. Therefore, it was a long time before elemental fluorine was isolated , and F. F. H. Moisson finally succeeded in isolating it by the electrolysis of KF in liquid HF. Fluorine is still manufactured by this reaction.
Chlorine, which is especially important in inorganic industrial chemistry, is manufactured together with sodium hydroxide. The basic reaction for the production of chlorine is electrolysis of an aqueous solution of NaCl using an ion exchange process. In this process, chlorine gas is generated in an anodic cell containing brine and Na+ moves through an ion exchange membrane to the cathodic cell where it pairs with OH- to become an aqueous solution of NaOH.
Why can chlorine be manufactured by electrolysis of an aqueous solution of sodium chloride?
- Answer
-
Despite the higher reduction potential of chlorine (+1.36 V) than that of oxygen (+1.23 V), the reduction potential of oxygen can be raised (overvoltage) depending on the choice of electrode used for the electrolysis process.
Bromine is obtained by the oxidation of Br- with chlorine gas in saline water. Iodine is similarly produced by passing chlorine gas through saline water containing I- ions. Since natural gas is found in Japan together with underground saline water containing I- Japan is one of the main countries producing iodine.
Anomalies of fluorine
Molecular fluorine compounds have very low boiling points. This is due to the difficulty of polarization as a result of the electrons being strongly drawn to the nuclei of fluorine atoms. Since the electronegativity of fluorine is highest (\(\chi\) = 3.98) and electrons shift to F, resulting in the high acidity of atoms bonded to F. Because of the small ionic radius of F-, high oxidation states are stabilized, and hence low oxidation compounds like CuF are unknown, in contrast with the compounds such as IF7 and PtF6.
Pseudohalogens
Since the cyanide ion CN-, the azide ion N3-, and the thiocyanate ion SCN-, etc. form compounds similar to those of halide ions, they are called pseudohalide ions. They form psudohalogen molecules such as cyanogene (CN)2, hydrogen cyanide HCN, sodium thiocyanate NaSCN, etc. Fine-tuning electronic and steric effects that are impossible with only halide ions make pseudohalogens useful also in transition metal complex chemistry.
Polyhalogens
Besides the usual halogen molecules, mixed halogen and polyhalogen molecules such as BrCl, IBr, ICl, ClF3, BrF5,IF7 etc also exist. Polyhalogen anions and cations such as I3-, I5-, I3+, and I5+, are also known.
(b) Oxygen compounds
Although many binary oxides of halogens (consisting only of halogen and oxygen) are known, most are unstable. Oxygen difluoride OF2 is the most stable such compound. This is a very powerful fluorinating agent and can generate plutonium hexafluoride PuF6 from plutonium metal. While oxygen chloride, Cl2O, is used for bleaching pulp and water treatment, it is generated in situ from ClO3-, since it is unstable.
Hypochlorous acid, HClO, chlorous acid, HClO2, chloric acid, HClO3, and perchloric acid, HClO4 are oxoacids of chlorine and especially perchloric acid is a strong oxidizing agent as well as being a strong acid. Although analogous acids and ions of other halogens had been known for many years, BrO4- was synthesized as late as 1968. Once it was prepared it turned out to be no less stable than ClO4- or IO4-, causing some to wonder why it had not been synthesized before. Although ClO4- is often used for crystallizing transition metal complexes, it is explosive and should be handled very carefully.
(c) Halides of nonmetals
Halides of almost all nonmetals are known, including fluorides of even the inert gases krypton, Kr, and xenon, Xe. Although fluorides are interesting for their own unique characters, halides are generally very important as starting compounds for various compounds of nonmetals by replacing halogens in inorganic syntheses (Table \(\PageIndex{8}\)).
| 1 | 2 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
|---|---|---|---|---|---|---|---|---|---|
| 2 | LiCl | BeCl2 | BF3 | CCl4 | NF3 | OF2 | |||
| 3 | NaCl | MgCl2 | AlCl3 | SiCl4 | PCl3 PCl5 |
S2Cl2 SF6 |
ClF3 ClF5 |
||
| 4 | KCl | CaCl2 | ZnCl2 | GaCl3 | GeF2 GeCl4 |
AsCl3 AsF5 |
Se2Cl2 SeF5 |
BrF3 BrF5 |
KrF2 |
| 5 | RbCl | SrCl2 | CdCl2 | InCl InCl3 |
SnCl2 SnCl4 |
SbCl3 SbF5 |
Te4Cl16 TeF6 |
IF5 IF7 |
XeF2 XeF6 |
| 6 | CsCl | BaCl2 | Hg2Cl2 HgCl2 |
TlCl TlCl3 |
PbCl2 PbCl4 |
BiCl3 BiF5 |
Boron trifluoride, BF3, is a colorless gas (mp -127 °C and bp -100 °C) that has an irritating odor and is poisonous. It is widely used as an industrial catalyst for Friedel-Crafts type reactions. It is also used as a catalyst for cationic polymerization. It exists in the gaseous phase as a triangular monomeric molecule, and forms Lewis base adducts with ammonia, amines, ethers, phosphines, etc. because of its strong Lewis acidity. Diethylether adduct, (C2H5)2O:BF3, is a distillable liquid and is used as a common reagent. It is a starting compound for the preparation of diborane, B2H6. Tetrafluoroborate, BF4-, is a tetrahedral anion formed as an adduct of BF3 with a base F-. Alkali metal salts, a silver salt and NOBF4 as well as the free acid HBF4 contain this anion. Since its coordination ability is very weak, it is used in the crystallization of cationic complexes of transition metals as a counter anion like ClO4-. AgBF4 and NOBF4 are also useful for 1-electron oxidation of complexes.
Tetrachlorosilane, SiCl4, is a colorless liquid (mp -70 °C and bp 57.6 °C). It is a regular tetrahedral molecule, and reacts violently with water forming silicic acid and hydrochloric acid. It is useful as a raw material for the production of pure silicon, organic silicon compounds, and silicones.
Phosphorus trifluoride, PF3, is a colorless, odorless, and deadly poisonous gas (mp -151.5 °C and bp -101.8 °C). This is a triangular pyramidal molecule. Because it is as electron-attracting as CO, it acts as a ligand forming metal complexes analogous to metal carbonyls.
Phosphorus pentafluoride, PF5, is a colorless gas (mp -93.7 °C and bp -84.5 °C). It is a triangular bipyramidal molecule and should have two distinct kinds of fluorine atoms. These fluorines exchange positions so rapidly that they are indistinguishable by 19F NMR. It was the first compound with which the famous Berry's pseudorotation was discovered as an exchange mechanism for axial and equatorial fluorine atoms (refer to Section 6.1). The hexafluorophosphate ion, PF6-, as well as BF4- is often used as a counter anion for cationic transition metal complexes. LiPF6 and R4NPF6 can be used as supporting electrolytes for electrochemical measurements.
Phosphorus trichloride, PCl3, is a colorless fuming liquid (mp -112 °C and bp 75.5 °C). It is a triangular pyramidal molecule and hydrolyzes violently. It is a soluble in organic solvents. It is used in large quantities as a raw material for the production of organic phosphorus compounds.
Phosphorus pentachloride, PCl5, is a colorless crystalline substance (sublimes but decomposes at 160 °C) It is a triangular bipyramidal molecule in the gaseous phase, but it exists as an ionic crystal [PCl4]+ [PCl6]- in the solid phase. Although it reacts violently with water and becomes phosphoric acid and hydrochloric acid, it dissolves in carbon disulfide and carbon tetrachloride. It is useful for cchlorination of organic compounds.
Arsenic pentafluoride, AsF5, is a colorless gas (mp -79.8 °C and bp -52.9 °C). It is a triangular bipyramidal molecule. Although it hydrolyzes, it is soluble in organic solvents. As it is a strong electron acceptor, it can form electron donor-acceptor complexes with electron donors.
Sulfur hexafluoride, SF6, is a colorless and odorless gas (mp -50.8 °C and sublimation point -63.8 °C) It is a hexacoordinate octahedral molecule. It is chemically very stable and hardly soluble in water. Because of its excellent heat-resisting property, incombustibility, and corrosion resistance, it is used as a high voltage insulator.
Sulfur chloride, S2Cl2, is an orange liquid (mp -80 °C and bp 138 °C). It has a similar structure to hydrogen peroxide. It is readily soluble in organic solvents. It is important as an industrial inorganic compound, and is used in large quantities for the vulcanization of rubber etc.
(d) Metal halides
Many metal halides are made by the combination of about 80 metallic elements and four halogens (Table \(\PageIndex{8}\), Table \(\PageIndex{9}\)). Since there are more than one oxidation state especially in transition metals, several kinds of halides are known for each transition metal. These halides are most important as starting materials of the preparation of metal compounds, and the inorganic chemistry of metal compounds depends on metal halides. There are molecular, 1-dimensional chain, 2-dimensional layer, and 3-dimensional halides but few of them are molecular in crystalline states. It should be noted that the anhydrous transition metal halides are usually solid compounds and hydrates are coordination compounds with water ligands. As the dimensionality of structures is one of the most interesting facets of structural or synthetic chemistry, typical halides are described in order of their dimensionality.
| Oxidation Number | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|
| +1 | ScCl YCl LaCl |
ZrCl HfCl |
CuCl AgCl AuCl |
||||||
| +2 | TiCl2 | VCl2 | CrCl2 MoCl2 WCl2 |
MnCl2 | FeCl2 RuCl2 |
CoCl2 | NiCl2 PdCl2 PdCl2 |
CuCl2 | |
| +3 | ScF3 YCl3 LaF3 |
TiCl3 ZrCl3 |
VCl3 | CrCl3 MoCl3 WCl3 |
ReCl3 | FeCl3 RuCl3 OsCl3 |
CoF3 RhCl3 IrCl3 |
AuCl3 | |
| +4 | TiCl4 ZrCl4 HfCl4 |
VCl4 NbCl4 TaCl4 |
CrF4 MoCl4 WCl4 |
ReCl4 | PtCl4 | ||||
| +5 | VF5 NbCl5 TaCl5 |
CrF5 MoCl5 WCl5 |
ReCl5 | OsF5 | IrF5 | PtF5 | |||
| +6 | ReF6 | OsF6 | IrF6 | PtF6 | |||||
| +7 | ReF7 | OsF7 |
Molecular halides
Mercury(II) chloride, HgCl2
It is a colorless crystal soluble in water and ethanol. It is a straight, three-atomic molecule in the free state. However, in addition to two chlorine atoms bonded to mercury, four additional chlorine atoms of adjacent molecules occupy coordination sites and the mercury is almost hexacoordinate in the crystalline state. The compound is very toxic and used for preserving wood, etc.
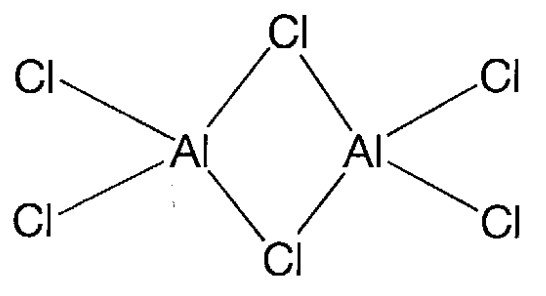
Aluminum trichloride, AlCl3.
A colorless crystal (mp 190 °C (2.5 atm) and bp 183 °C) that sublimes when heated. It is soluble in ethanol and ether. It is a Lewis acid and forms adducts with various bases. It is a molecule consisting of the dimer of tetracoordinate aluminium with chlorine bridges in the liquid and gaseous phases (Figure \(\PageIndex{21}\)), and takes a lamellar structure when crystalline. It is used as a Lewis acid catalyst of Friedel-Crafts reactions, etc.
Tin (IV) chloride, SnCl4
A colorless liquid (mp -33 °C and bp 114 °C). In the gaseous state, it is a tetrahedral molecule.
Titanium(IV) chloride, TiCl4
A colorless liquid (mp -25 °C and bp 136.4 °C). The gaseous molecule is a tetrahedron similar to tin(IV) chloride. It is used as a component of the Ziegler Natta catalyst (refer to Section 8.1 (a)).
Chain-like halides
Gold (I) iodide, AuI
Yellow white solid. Two iodines coordinate to gold, and the compound has a zigzag 1-dimensional chain structure.
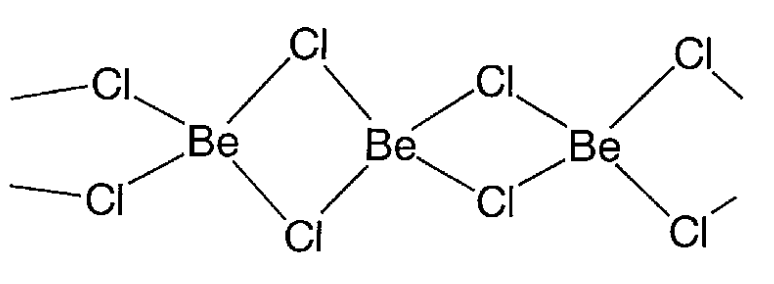
Beryllium chloride, BeCl2
A colorless crystal (mp 405 °C and bp 520 °C). It is deliquescent and soluble in water and ethanol. The tetra-coordinated beryllium forms a 1-dimensional chain via chlorine bridges (Figure \(\PageIndex{22}\)). In the gaseous phase, it is a straight two-coordinate molecule. It is a Lewis acid and is used as a catalyst for Friedel-Crafts reactions.
Palladium chloride, PdCl2
A dark red solid. In the \(\alpha\) type, the four-coordinate palladium forms a 1-dimensional chain with double bridges of chlorines. The dihydrate is deliquescent and soluble in water, ethanol, acetone, etc. When it is dissolved in hydrochloric acid, it becomes four-coordinate square-planar [PdCl4]2-. It is used as the catalyst for the Wacker process, which is an olefin oxidation process, or in various catalysts for organic syntheses.
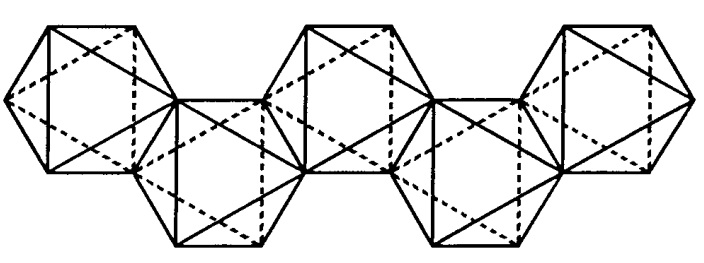
Zirconium tetrachloride, (IV) ZrCl4
A colorless crystal (it sublimes above 331 °C). The zirconium is octahedrally coordinated and forms a zigzag chain via chlorine bridges (Figure \(\PageIndex{23}\)). It is hygroscopic and soluble in water, ethanol, etc. It is used as a Friedel-Crafts catalyst and as a component of olefin polymerization catalysts.
Stratified halides
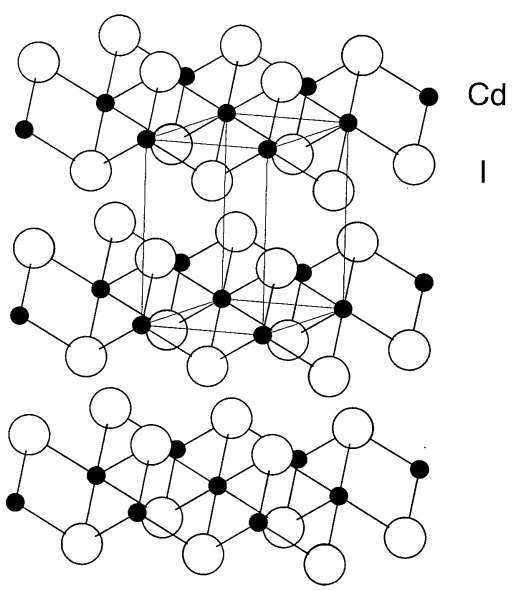
Cadmium iodide, CdI2
A colorless crystal (mp 388 °C and bp 787 °C). It has a cadmium iodide structure where the layers of edge-shared CdI6 octahedral units are stratified (Figure \(\PageIndex{24}\)). In the gaseous phase, it comprises straight three atomic molecules. It dissolves in water, ethanol, acetone, etc.
Cobalt(II) chloride, CoCl2
Blue crystals (mp 735 °C and bp 1049 °C). It has the cadmium chloride structure. It is hygroscopic and becomes light red when water is absorbed. It is soluble also in ethanol and acetone. The hexahydrate is red and is a coordination compound in which water molecules are ligands.
Iron (II)chloride, FeCl2
Greenish yellow crystals (mp 670-674 °C). It has the cadmium chloride structure, and is soluble in water and ethanol. The hydrates, which are coordinated by various numbers (6, 4, 2) of water molecules, are precipitated from aqueous solutions of hydrochloric acid.
Iron(III) chloride, FeCl3
Dark brown crystals (mp 306 °C and sublimes). It has a lamellar structure in which iron is octahedrally surrounded by six chlorine ligands. In the gaseous phase, it has a dimeric structure bridged by chlorine atoms similar to that of aluminum chloride.
3-dimensional structure halides
Sodium chloride, NaCl
A colorless crystal (mp 801 °C and bp 1413 °C). It is the original rock salt-type structure. In the gaseous phase, this is a two-atom molecule. Although it is soluble in glycerol as well as water, it hardly dissolves in ethanol. Large single crystals are used as prisms for infrared spectrometers.
Cesium chloride, CsCl
A colorless crystal (mp 645 °C, bp 1300 °C). Although it has the cesium chloride type structure, it changes to the rock salt structure at 445 °C. In the gaseous phase, it is a two-atom molecule.
Copper(I) chloride, CuCl
A colorless crystal (mp 430 °C and bp 1490 °C) It has the zinc blende structure and four chlorines tetrahedrally coordinate to copper.
Calcium chloride, CaCl2
A colorless crystal (mp 772 °C and bp above 1600 °C). It has a deformed rutile-type structure and calcium is octahedrally surrounded by six chlorines. It is soluble in water, ethanol, and acetone. It is deliquescent and used as a desiccant. Hydrates in which 1, 2, 4, or 6 water molecules are coordinated are known.
Calcium fluoride, CaF2
A colorless crystal (mp 1418 °C and bp 2500 °C). It has the fluorite type structure. It is the most important raw material for fluorine compounds. Good quality crystals are used also as spectrometer prisms and in photographic lenses.
Chromium(II) chloride, CrCl2
A colorless crystal (mp 820 °C and sublimes). It has a deformed rutile-type structure. It dissolves well in water giving a blue solution.
Chromium(III) chloride, CrCl3
Purplish red crystal (mp 1150 °C and decomposes at 1300 °C). Cr3+ occupies two thirds of the octahedral cavities in every other layer of Cl- ions, which are hexagonally close-packed. It is insoluble in water, ethanol, and acetone.
Why do solid metal halides dissolve in water?
- Answer
-
It is because water reacts with halides breaking the halogen bridges in the solid structures and coordinates to the resultant molecular complexes.


